Your Location:Home > Products > Cosmetic and Beauty Materials > L-Carnosine


CasNo: 305-84-0
MF: C9H14N4O3
Appearance: crystalline
|
Reference |
P. J. Quinn, A. A. Boldyrev, V. E. Formazuyk, Carnosine: Its properties, functions and potential therapeutic applications, Molecular Aspects of Medicine, 1992, vol. 13, pp. 379-444 https://www.webmd.com/vitamins/ai/ingredientmono-1038/carnosine G. M. Halpern, Zinc-Carnosine: Nature’s Safe and Effective Remedy for Ulcers, 2005, ISBN-10 0757002749 |
|
benefits |
L-Carnosine is a strong anti-glycosylation, free radical scavenging,anti-oxidant,anti-aging, anti-pollution.Brightenand repairthe skin. white powder.Its recommended dosage is 0.05~2%. |
|
Synthesis Reference(s) |
The Journal of Organic Chemistry, 48, p. 392, 1983 DOI: 10.1021/jo00151a026 |
|
Flammability and Explosibility |
Notclassified |
|
Biochem/physiol Actions |
L-Carosine is a dipeptide found at millimolar concentration in brain, muscle and the lens of the eye. In model systems it is a potent antioxidant that scavenges oxygen free radicals and transition metal ions. It blocks protein-protein and protein-DNA cross-links induced by hypochlorite anions and toxic aldehydes such as acetaldehyde, formaldehyde, and malondialdehyde, the primary product of lipid peroxidation. It also inhibits nonenzymatic protein glycation induced by aldose and ketose reducing sugars and inhibits the formation of toxic advanced glycation end products (AGE). These activities make it of interest in studies of aging, atherosclerosis, Alzheimer′s disease, and the secondary effects of diabetes. |
|
Safety Profile |
Mildly toxic by intraperitoneal route. An experimental teratogen. Other experimental reproductive effects. When heated to decomposition it emits toxic fumes of NOx. |
|
Purification Methods |
Likely impurities are histidine and β-alanine. Crystallise L-carnosine from water by adding EtOH in excess. Recrystallise it from aqueous EtOH by slow addition of EtOH to a strong aqueous solution of the dipeptide. Its solubility in H2O is 33.3% at 25o. [Vinick & Jung J Org Chem 48 392 1983, Turner J Am Chem Soc 75 2388 1953, Sifford & du Vigneaud J Biol Chem 108 753 1935, Beilstein 25 H 516, 25 I 717, 25 II 408.] |
|
Chemical Composition and Structure |
Carnosine, also known as β-alanyl-L-histidine, is a dipeptide composed of the amino acids β-alanine and L-histidine. It is characterized by its low molecular weight and hydrophilic nature. |
|
Sources |
Carnosine is found endogenously in body tissues, particularly in skeletal muscle, where its concentration is higher than in any other tissue. It is also present in other excitable tissues. |
|
Definition |
ChEBI: A dipeptide that is the N-(beta-alanyl) derivative of L-histidine. |
InChI:InChI=1/C9H14N4O3/c10-2-1-8(14)13-7(9(15)16)3-6-4-11-5-12-6/h4-5,7H,1-3,10H2,(H,11,12)(H,13,14)(H,15,16)/t7-/m0/s1
Carnosine (β-alanyl-L-histidine) and hom...
-
-
-
Solid-phase peptide synthesis of dipepti...
The invention provides a carnosine inter...
Disclosed are methods and compositions o...
l-Carnosine (l-Car, β-alanyl-l-histidine...
The invention relates to a method for pr...
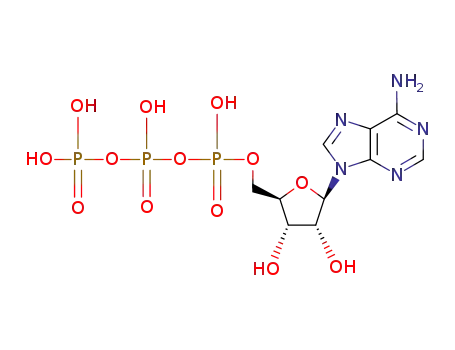
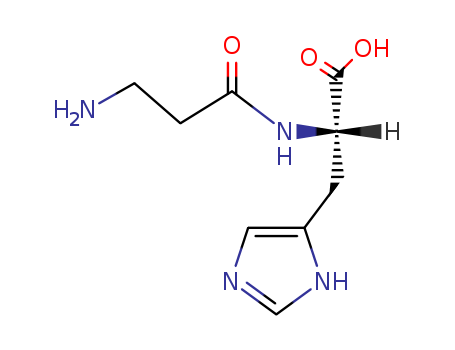
ATP

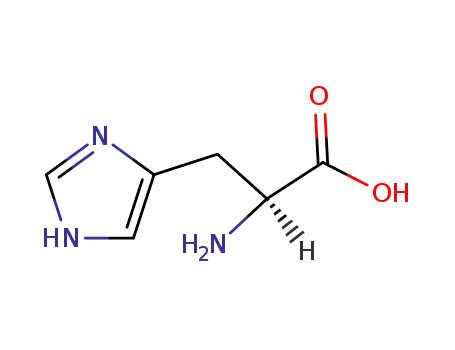
L-histidine

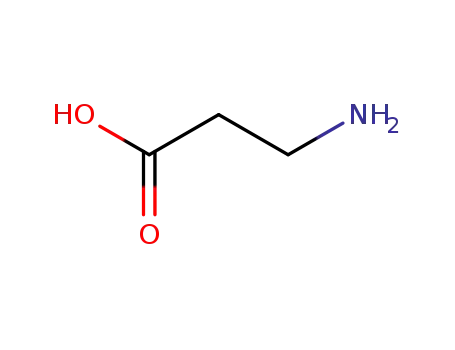
3-amino propanoic acid

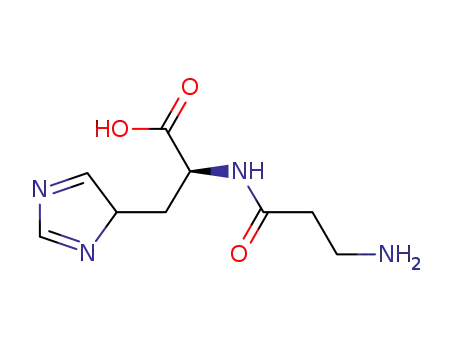
carnosine

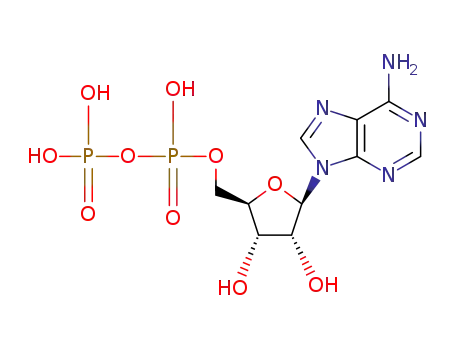
adenosine 5'-diphosphate
| Conditions | Yield |
|---|---|
|
With
ethylene glycol-bis(2-aminoethyl)-N,N,N'N,'-tetraacetic acid; chicken carnosine synthase; magnesium chloride; diothiothreitol;
at 37 ℃;
for 0.333333h;
pH=7.5;
aq. buffer;
Enzymatic reaction;
|
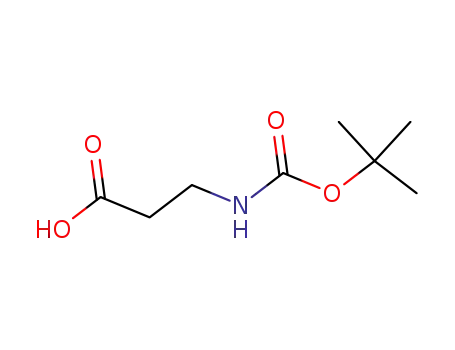
3-(tert-butyloxycarbonylamino)propionic acid

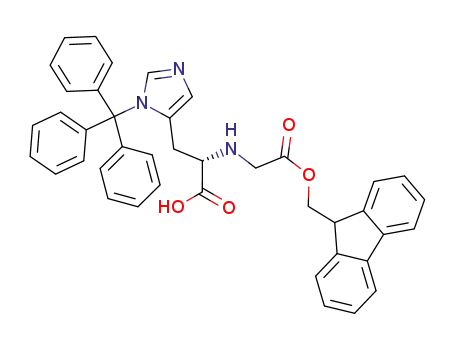
N-9-fluorenylmethyloxycarbonyl-N-trityl-L-histidine

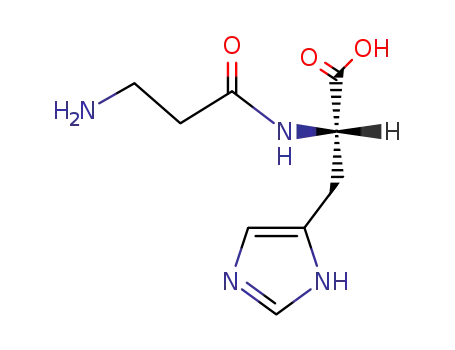
carnosine
| Conditions | Yield |
|---|---|
|
N-9-fluorenylmethyloxycarbonyl-N-trityl-L-histidine;
With
trityl chloride polystyrene resin; N-ethyl-N,N-diisopropylamine;
In
dichloromethane; N,N-dimethyl-formamide;
for 0.5h;
trityl chloride resin
With
piperazine;
In
dichloromethane; N,N-dimethyl-formamide;
for 1h;
trityl chloride resin
Inert atmosphere;
3-(tert-butyloxycarbonylamino)propionic acid;
Further stages;
|
90% |
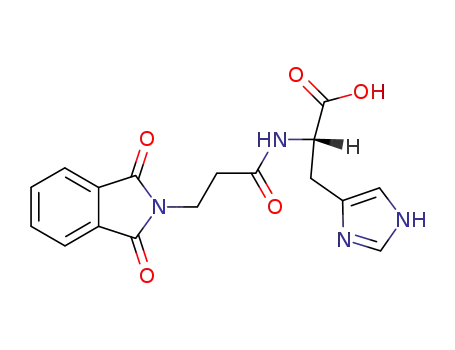
Nα-(N,N-phthaloyl-β-alanyl)-histidine
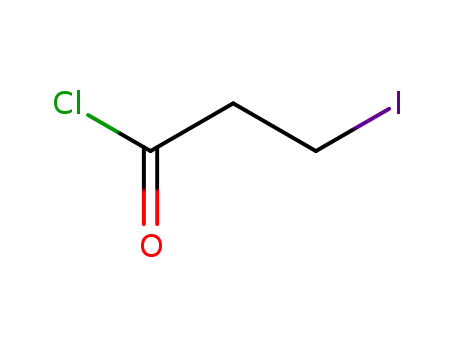
3-iodopropionyl chloride

L-histidine

3-amino propanoic acid
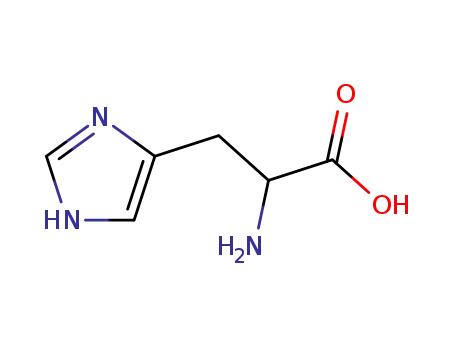
D,L-histidine

3-amino propanoic acid
4-toluenesulfonylureido-carnosine

L-histidine