Your Location:Home > Products > Food Ingredients > L-Malic Acid


CasNo: 97-67-6
MF: C4H6O5
Appearance: clear colourless solution
|
Preparation |
L-Malic acid can be prepared by hydration of maleic acid; by fermentation from sugar. |
|
Biochem/physiol Actions |
L-Malic acid is a part of cellular metabolism. Its application is recognized in pharmaceutics. It is useful in the treatment of hepatic malfunctioning, effective against hyper-ammonemia. It is used as a part of amino acid infusion. L-Malic acid also serves as a nanomedicine in the treatment of brain neurological disorders. A TCA (Krebs cycle) intermediate and partner in the malic acid aspartate shuttle. |
|
Purification Methods |
Crystallise S-malic acid (charcoal) from ethyl acetate/pet ether (b 55-56o), keeping the temperature below 65o. Or dissolve it by refluxing in fifteen parts of anhydrous diethyl ether, decant, concentrate to one-third volume and crystallise it at 0o, repeatedly to constant melting point. [Beilstein 3 IV 1123.] |
|
Definition |
ChEBI: An optically active form of malic acid having (S)-configuration. |
|
General Description |
L-Malic acid?is an organic acid that is commonly found in wine. It plays an important role in wine microbiological stability. |
InChI:InChI=1/C4H6O5/c5-2(4(8)9)1-3(6)7/h2,5H,1H2,(H,6,7)(H,8,9)/t2-/m0/s1
11 new sesquiterpene dimers, sarglabolid...
Mercurialis annua and M. perennis are me...
The instant invention describes methods ...
Malic enzyme [L-malate: NAD(P)+ oxidored...
![2-[(E)-3-(3,4-dihydroxyphenyl)prop-2-enoyl]oxybutanedioic acid](/upload/2025/8/2fe66aa2-884f-41f3-9220-b96dbe1f4e94.png)
2-[(E)-3-(3,4-dihydroxyphenyl)prop-2-enoyl]oxybutanedioic acid

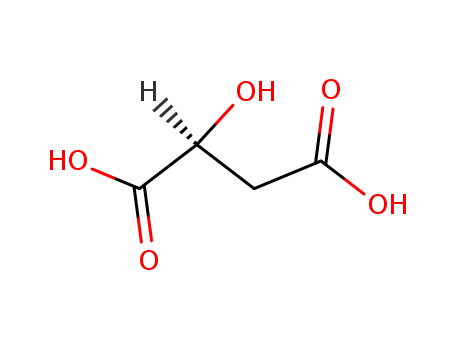
D-Malic acid

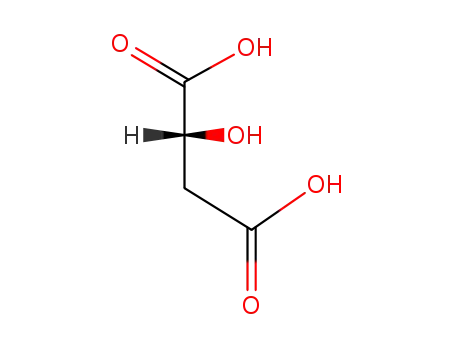
(S)-Malic acid
| Conditions | Yield |
|---|---|
|
2-[(E)-3-(3,4-dihydroxyphenyl)prop-2-enoyl]oxybutanedioic acid;
With
sodium hydroxide;
In
water;
for 24h;
With
hydrogenchloride;
In
water;
pH=1.2;
|
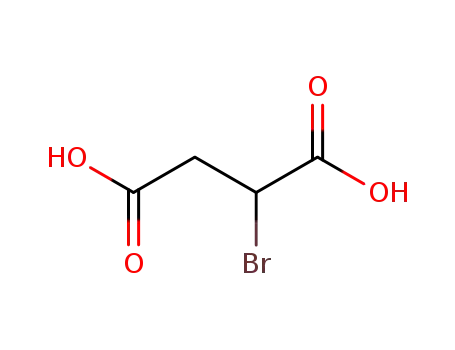
bromosuccinic acid


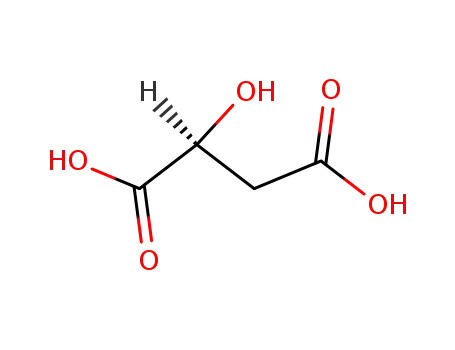
D-Malic acid


(S)-Malic acid
| Conditions | Yield |
|---|---|
|
With
alkali;
Hydrolysis.bei der Hydrolyse der Kupfer(II)-salze;
|
|
|
With
water;
Hydrolysis.bei der Hydrolyse der Kupfer(II)-salze;
|
|
|
Hydrolysis.in saurer oder anfangs neutraler Loesung;
|
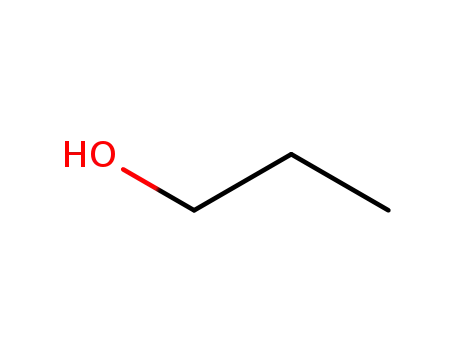
propan-1-ol
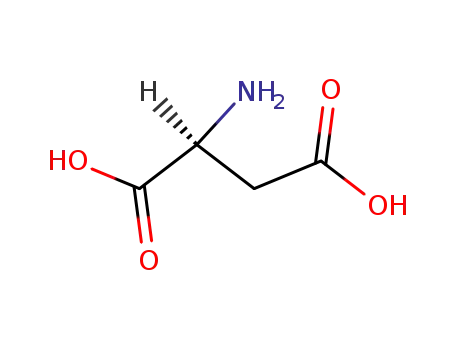
L-Aspartic acid
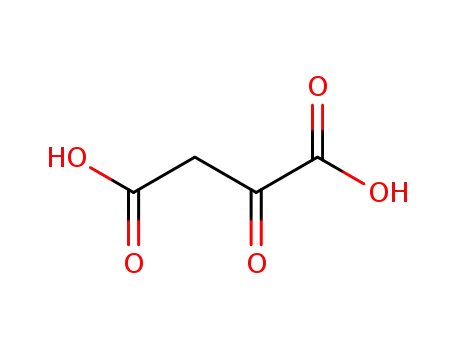
Oxalacetic acid
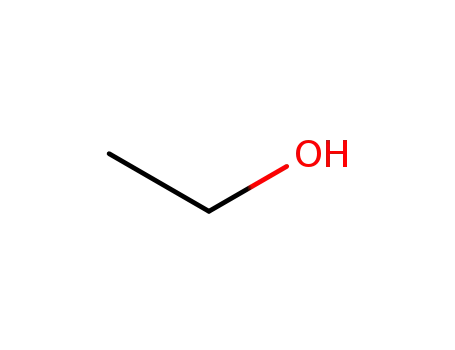
ethanol
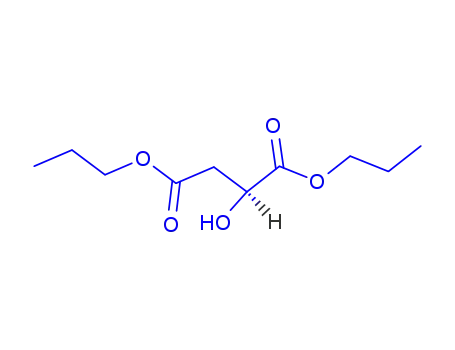
(S)-(-)-di-n-propyl 2-hydroxybutandioate

Oxalacetic acid
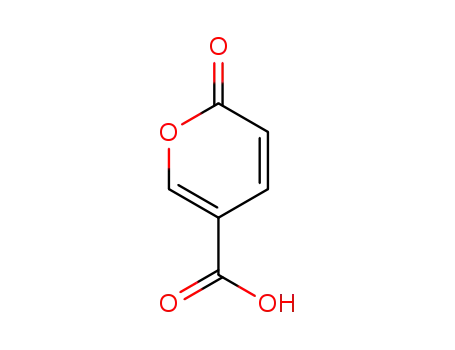
2H-pyran-2-one-5-carboxylic acid
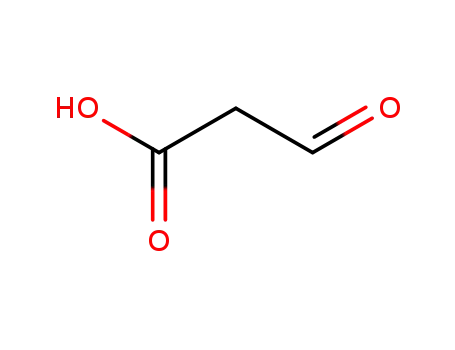
3-oxopropanoic acid