Your Location:Home > Products > Food ingredients > L-Aspartic acid


CasNo: 56-84-8
MF: C4H7NO4
Appearance: White crystalline powder
|
Production |
L- aspartic acid is mainly produced by enzymatic method. L- aspartase acts on the fumaric acid and ammonia, that is, which generates L- aspartic acid. Strain Culturing: Eschrichia coli Asl.881 was cultured. The common meat juice medium is agarslantculture-medium. The vase medium comprises corn steep liquor 7.5%, fumaric acid 2.0% and MgSO4 7H2O 0.02%. Adjust the pH value of solution to 6.0 with ammonia water, then put 50-100ml culture medium into 500ml cone bottle after boiling and filtering. Take the fresh cultivated seeds on the slope or in the liquid, inoculate culture medium in shake flask, shake overnight at 37℃, adjust pH to 5.0 with 1mol/L HCl after enlarging culture step by step to 1000-2000L, cool it to room temperature after keeping 45℃ for 1h, centrifuge in rotary supercentrifuge and collect the thallus including aspartase. Immobilize aspartase: Make a bioreactor to take out 20kg E.Coli wet cell, suspend it in the culture supernatant after centrifugation in 80L (or 80L saline), keep it at 40℃ and then add 90L 12% gelatin solution and 1.0% glutaraldehyde solution, which should be held 40℃. ?Stir well, set aside to cool down and solidify, soak in 0.25% glutaraldehyde solution, hold 5℃ after an overnight, cut into small pieces ( 3-5 mm3) , soak in 0.25% glutaraldehyde solution at 5℃ for a night, take it out and elut with water, drain to obtain immobilized E.Coli containing aspartase and load it into filled bioreactor in reserve. Conversion: The solution of 1 mol/L of ammonium fumarate (including 1mmol/L MgCl2, pH8.5) substrate, which keeps 37℃, flows through a bioreactor at a constant speed (SV) continuously in the case of controlling the maximum conversion rate over 95% and then the conversion solution is obtained. Roughhew and refine: ?Add 1 mol/L of HCl into the conversion solution gradually to adjust pH valkue to 2.8, place at 5℃ overnight for crystallizing, filter to prepare crystallization, drain after water washing, drying at 105℃ to obtain L- aspartic acid crude. Use dilute ammonia to recrystallize, dissolve into 15% solution (pH5.0) with ammonia, add 1% activated carbon, stir and fade for 1h when heat to 70℃, filter immediately to remove slag, cool the filtrate, hold 5℃ overnight for crystallizing, filter to get crystallization and obtain the L-Aspartic acid finished products after vacuum drying at 85℃. |
|
History |
Aspartic acid was first discovered in 1827 by Plisson, derived from asparagine, which had been isolated from asparagus juice in 1806, by boiling with a base. |
|
Hazard |
Low toxicity. |
|
Biological Activity |
Endogenous NMDA receptor agonist. |
|
Safety Profile |
Low toxicity by intraperitoneal route. When heated to decomposition emits toxic fumes of NOx. |
|
Synthesis |
Enzymatically, aspartic acid is reversibly synthesized by a transamination reaction between oxaloacetic acid and glutamic acid in the presence of pyridoxal phosphate. |
|
Forms and nomenclature |
There are two forms or enantiomers of aspartic acid. The name "aspartic acid" can refer to either enantiomer or a mixture of two. Of these two forms, only one, "L - aspartic acid", is directly incorporated into proteins. The biological roles of its counterpart, "Daspartic acid" are more limited. Where enzymatic synthesis will produce one or the other, most chemical syntheses will produce both forms, "DL-aspartic acid," known as a racemic mixture. |
|
Other biochemical roles |
Aspartate is also a metabolite in the urea cycle and participates in gluconeogenesis. It carries reducing equivalents in the malateaspartate shuttle, which utilizes the ready inter conversion of aspartate and oxaloacetate, which is the oxidized (dehydrogenated) derivative of malic acid. Aspartate donates one nitrogen atom in the biosynthesis of inosine, the precursor to the purine bases. In addition, aspartic acid acts as hydrogen acceptor in a chain of ATP synthase. |
|
Nature and Forms |
Aspartic acid exists in two enantiomeric forms: L-aspartic acid and D-aspartic acid. |
|
Constituent of Proteins |
Aspartic acid is one of the top three main constituents of proteins. |
|
Bio functionality and Uses |
Aspartic acid is an acidic amino acid that can chelate or adsorb metal ions. |
|
Market and Production |
The global aspartic acid market consists of several small company players and is growing, particularly in the medical sector. |
|
Industrial Relevance and Growth |
Aspartic acid has significant potential for industrial relevance and is expected to see increased demand, particularly in the medical and beverage sectors in regions like Asia Pacific. |
|
General Description |
L-Aspartic acid, also known by various synonyms such as L-Aspartate or (S)-Aspartic acid, is a non-essential amino acid that plays a critical role in the urea cycle and gluconeogenesis. It serves as a key intermediate in metabolic pathways, including the synthesis of other amino acids, nucleotides, and neurotransmitters. L-Aspartic acid is also involved in energy production through its participation in the citric acid cycle and acts as a neurotransmitter in the central nervous system. Its (S)-configuration is biologically active, contributing to protein structure and enzymatic functions. Additionally, it has applications in food, pharmaceutical, and supplement industries due to its nutritional and physiological significance. |
|
Definition |
ChEBI: The L-enantiomer of aspartic acid. |
InChI:InChI=1/C4H7NO4/c5-2(4(8)9)1-3(6)7/h2H,1,5H2,(H,6,7)(H,8,9)/t2-/m0/s1
Surfactins are natural biosurfactants wi...
N-demethylases have been reported to rem...
In industry,l-alanine is biosynthesized ...
Malaria remains a worldwide threat, affl...
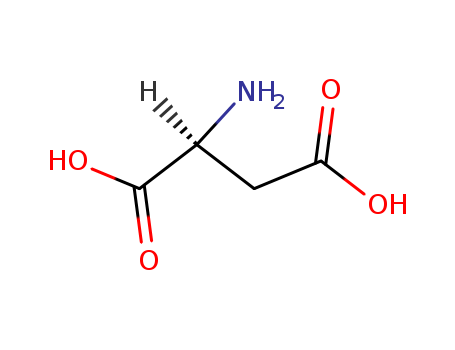
aspartic Acid

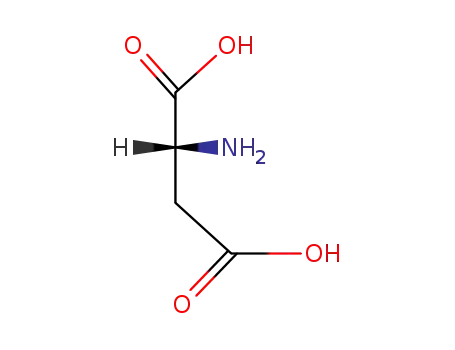
(2R)-aspartic acid

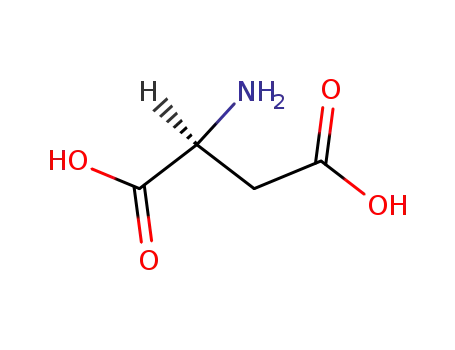
L-Aspartic acid
| Conditions | Yield |
|---|---|
|
With
L-alanin;
In
water;
for 24h;
Product distribution;
Ambient temperature;
other optically active amino acids;
|
|
|
With
teicoplanin;
In
methanol; water;
Further Variations:;
Reagents;
pH-values;
Solvents;
Product distribution;
|
|
|
With
(2R,3R,11R,12R)-(+)-18-crown-6-2,3,11,12-tetracarbonic acid;
|
|
|
With
L-arginine impregnated silica-G gel plates;
In
water; acetic acid; butan-1-ol;
pH=4;
Resolution of racemate;
|
|
|
With
acetone;
In
water;
at 25 ℃;
Reagent/catalyst;
Concentration;
Resolution of racemate;
|
|
|
With
oxygen; sodium hydroxide;
In
water;
at 70 ℃;
for 4h;
pH=9;
Time;
pH-value;
Reagent/catalyst;
Temperature;
Catalytic behavior;
|
> 99.5 % ee |
|
With
sodium dihydrogenphosphate; calcium chloride;
In
water;
for 144h;
pH=5.6;
undersaturated dynamic brushite
Reagent/catalyst;
Resolution of racemate;
|
12 % ee |
|
With
R-(3,3'-dibromo-1,1'-binaphthyl)-20-crown-6 coated C18 silica gel column;
at 25 ℃;
pH=2;
Resolution of racemate;
|
|
|
With
lysozyme loaded covalent organic framework-1;
In
aq. phosphate buffer; isopropyl alcohol;
pH=6.5;
Reagent/catalyst;
Resolution of racemate;
Enzymatic reaction;
|
|
|
With
capillary electrochromatography open-tubular column coated with 1-allylimidazolium-β-cyclodextrin;
In
aq. acetate buffer;
at 20 ℃;
pH=8;
pH-value;
Resolution of racemate;
|
![{[1-{2-[((S)-1-Benzyl-pyrrolidine-2-carbonyl)-amino]-phenyl}-1-phenyl-meth-(E)-ylidene]-amino}-bromo-acetic acid](/upload/2025/8/dc75b2f0-13c6-4cac-a88c-78ef05158682.png)
{[1-{2-[((S)-1-Benzyl-pyrrolidine-2-carbonyl)-amino]-phenyl}-1-phenyl-meth-(E)-ylidene]-amino}-bromo-acetic acid

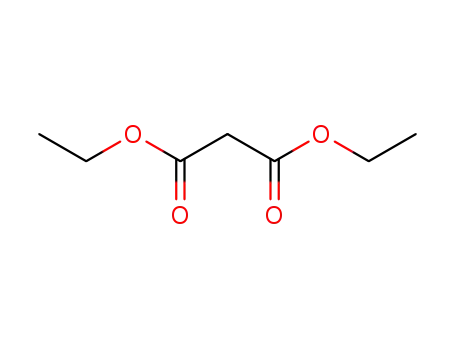
diethyl malonate


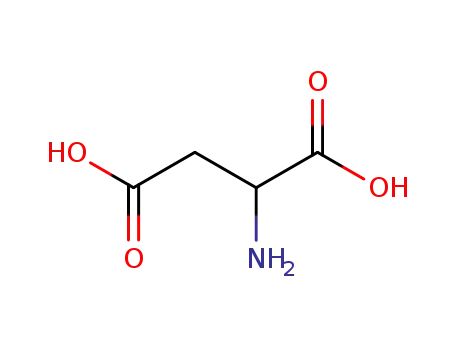
(2R)-aspartic acid


L-Aspartic acid
| Conditions | Yield |
|---|---|
|
With
hydrogenchloride; potassium tert-butylate; bromine; triethylamine; nickel dichloride;
Yield given. Multistep reaction;
1.) iPrOH, 5 deg C, 2.) CH3CN, -50 deg C, 3.) 25 deg C, H2O, reflux, 1 h;
|
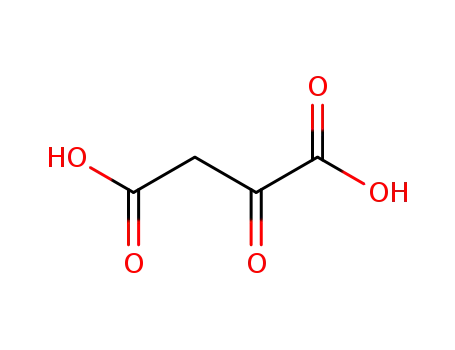
Oxalacetic acid
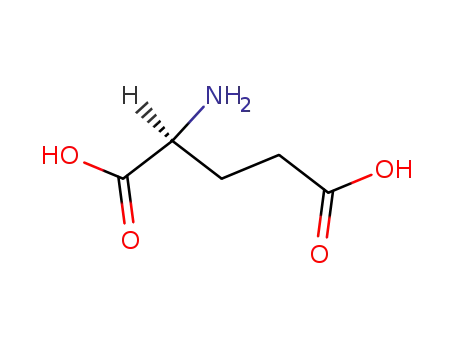
L-glutamic acid
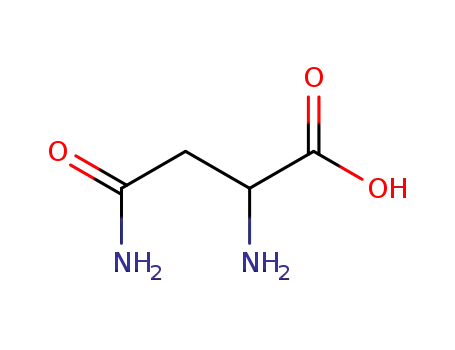
ASPARAGINE
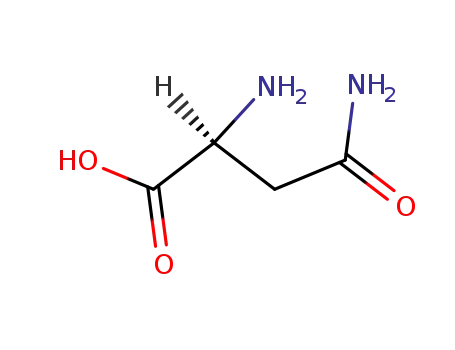
L-asparagine
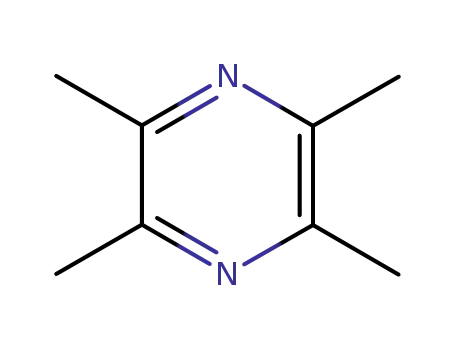
Tetramethylpyrazine
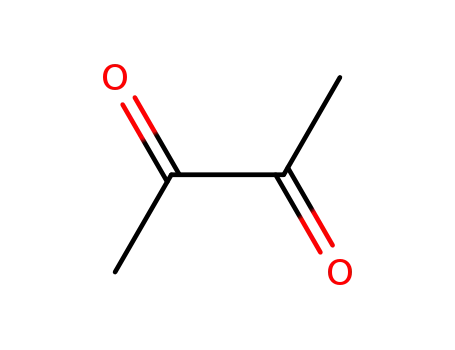
dimethylglyoxal
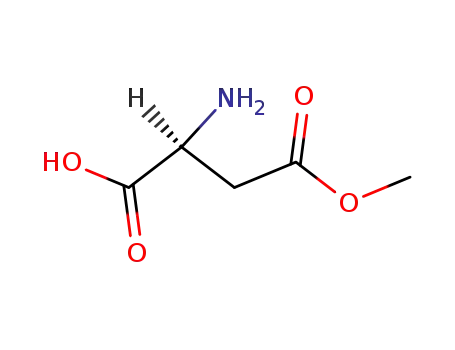
β-methyl L-aspartate
(+)-β-methyl-L-aspartate hydrochloride