Your Location:Home > Products > Herbs Extract and Health Care > Gallic Acid
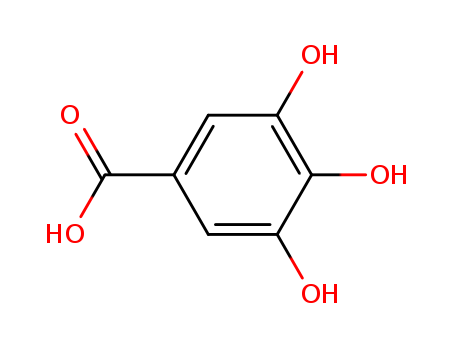


CasNo: 149-91-7
MF: C7H6O5
Appearance: white crystalline powder
|
Biotechnological Production |
The production of gallic acid is challenging. Conventionally, it has been produced by acid hydrolysis of tannic acid. However, this process is expensive due to low yields and high impurities. To overcome this problem, microbial production of gallic acid has been suggested. For example, in a solid-state fermentation of Teri pod cover powder containing tannin using Rhizopus oryzae, a yield of 90.9 % based on the tannin content of 58 % of the substrate was observed. In a submerged culture of Aspergillus aceleatus DBF9 growing on a medium with 3 % tannin, a maximal product concentration of 6.8 g.L-1 was reported. With tannic acid, even higher product concentrations of up to 25 g.L-1, a yield of 0.83 g of gallic acid per gram of tannic acid, and a productivity of 0,56 g.L-1.h-1 were shown using Apergillus fischeri MTCC 150 in submerged cultivation. An alternative is the enzymatic hydrolysis of tannic acids using tannase produced by microorganisms (e.g. Aspergillus fischeri or R. oryzae). For example, propyl gallate could be produced using a tannase from Emericela nidulans immobilized on ionic and covalent supports. |
|
Air & Water Reactions |
Sparingly water soluble |
|
Reactivity Profile |
Phenols, such as Gallic acid, do not behave as organic alcohols, as one might guess from the presence of a hydroxyl (-OH) group in their structure. Instead, they react as weak organic acids. Phenols and cresols are much weaker as acids than common carboxylic acids (phenol has Ka = 1.3 x 10^[-10]). These materials are incompatible with strong reducing substances such as hydrides, nitrides, alkali metals, and sulfides. Flammable gas (H2) is often generated, and the heat of the reaction may ignite the gas. Heat is also generated by the acid-base reaction between phenols and bases. Such heating may initiate polymerization of the organic compound. Phenols are sulfonated very readily (for example, by concentrated sulfuric acid at room temperature). The reactions generate heat. Phenols are also nitrated very rapidly, even by dilute nitric acid. |
|
Health Hazard |
Inhalation of dust may irritate nose and throat. Contact with eyes or skin causes irritation. |
|
Fire Hazard |
Flash point data for Gallic acid are not available. Gallic acid is probably combustible. |
|
Flammability and Explosibility |
Notclassified |
|
Biochem/physiol Actions |
Gallic acid is a water soluble phenolic acid present in grapes and in the leaves of many plants. Gallic acid esters, such as tannins, catechin gallates and aliphatic gallates are potent antioxidants in vitro. However, gallic acid itself also appears to have antioxidant, anticarcinogenic and antiangiogenic activity in vitro. |
|
Side effects |
It is a weak carbonic anhydrase inhibitor. |
|
Metabolism |
Biosynthesis Chemical structure of 3,5- didehydro shikimate Gallic acid is formed from 3-dehydro shikimate by the action of the enzyme shikimate dehydro genase to produce 3,5-didehydro shikimate. This latter compound tautomerizes to form the redox equivalent gallic acid, where the equilibrium lies essentially entirely toward gallic acid because of the coincidently occurring aromatization. Degradation Gallate dioxygenase is an enzyme found in Pseudomonas putida that catalyzes the reaction : gallate + O2 → (1E)-4-oxobut-1-ene-1,2,4-tri carboxylate. Gallate decarboxylase is another enzyme in the degradation of gallic acid. Conjugation Gallate 1-beta-glucosyltransferase is an enzyme that uses UDPglucose and gallate, whereas its two products are UDP and 1-galloylbeta- D-glucose. |
|
Purification Methods |
Crystallise gallic from water. The tri-O-acetyl derivative has m 172o (from MeOH), and the anilide has m 207o(from EtOH). [Beilstein 10 H 470, 10 IV 1993.] |
|
Esters |
Also known as galloylated esters: Methyl gallate Ethyl gallate, a food additive with E number E313 Propyl gallate, or propyl 3,4,5-trihydroxybenzoate, an ester formed by the condensation of gallic acid and propanol Octyl gallate, the ester of octanol and gallic acid Dodecyl gallate, or lauryl gallate, the ester of dodecanol and gallic acid Epicatechin gallate, a flavan-3-ol, a type of flavonoid, present in green tea Epigallocatechin gallate (EGCG), also known as epigallocatechin 3-gallate, the ester of epigallocatechin and gallic acid, and a type of catechin Gallocatechin gallate (GCG), the ester of gallocatechin and gallic acid and a type of flavan-3ol Theaflavin-3-gallate, a theaflavin derivative. |
|
General Description |
Gallic acid is a trihydroxybenzoic acid derivative with notable pharmacological properties, including potential roles as a liver-protective agent, an antidiabetic compound (as seen in its involvement in penta-O-galloyl-D-glucopyranose), and an inhibitor of α-synuclein aggregation in neurodegenerative diseases. Its structure allows for chemical modifications, such as amide derivatization, to enhance lipophilicity and blood-brain barrier penetration, improving therapeutic efficacy. Additionally, gallic acid serves as a key intermediate in synthesizing bioactive compounds, demonstrating its versatility in medicinal chemistry and material science applications. |
|
Definition |
ChEBI: A trihydroxybenzoic acid in which the hydroxy groups are at positions 3, 4, and 5. |
InChI:InChI=1/C7H6O5/c8-4-1-3(7(11)12)2-5(9)6(4)10/h1-2,8-10H,(H,11,12)
Phenolic compounds from leafy shoots of ...
An aqueous solution of the roots of the ...
To understand the stability of oolongthe...
Amariin is an ellagitannin with two dehy...
Nine hydrolyzable tannins, including thr...
Phytochemical investigations of the root...
Tannase of Enterobacter sp. was purified...
Chemical and biological investigations o...
In a phytochemical and chemotaxonomical ...
An investigation of the bark of Myrica r...
A new polyphenol, 6-O-galloylhomoarbutin...
The proportion of ingested anthocyanins ...
A new biflavonoid, semecarpuflavanone, h...
The polyphenolic compound 1-O-galloyl-3,...
With the elaboration of high-yielding, h...
A novel diacylglycerol-based galloyl str...
Three ellagitannins and one disulfated f...
-
Two new compounds that were identified a...
Gallic acid acts as a precursor molecule...
-
Key Word Index - Quercus stenophylla; Fa...
C-Glucosidic ellagitannin dimers were cl...
Polyflavanostilbene B (1), an unusual ad...
Malabathrins A (6), E (11) and F (14), n...
A new isocoumarin (1) named fraxicoumari...
A polystyrene-hollow sphere catalyst was...
Three new diarylheptanoids, myricanol 11...
-
Two novel glycosides, 4,5-dimethoxy-3-hy...
In the research on ellagitannin metaboli...
-
Three new bergenin derivatives were isol...
Key Word Index - Saxifraga stolonifera; ...
A new flavonol galactopyranoside, myrice...
Four new dimeric hydrolyzable tannins, h...
The new compounds nilocitin (2,3-digallo...
Five new hydrolyzable tannin dimers, ros...
This paper presents a new application fo...
Bioassay-guided fractionation of the MeO...
N-ethyl-2-pyrrolidinone-substituted flav...
The first investigation of Phyllanthus m...
(?)‐Epigallocatechin gallate (EGCG), the...
Soil microbes are an abundant source of ...
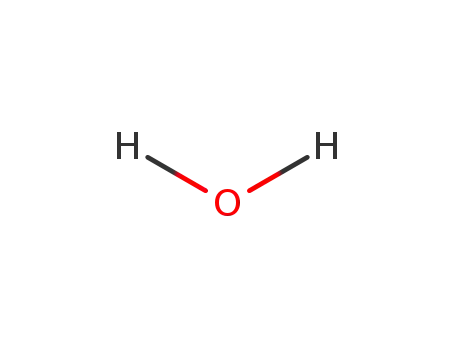
water

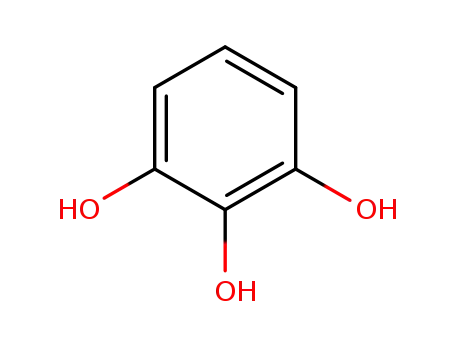
2-hydroxyresorcinol

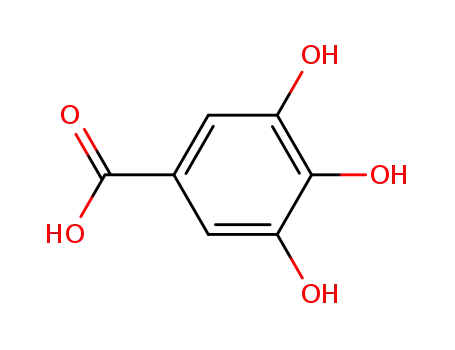
3,4,5-trihydroxybenzoic acid

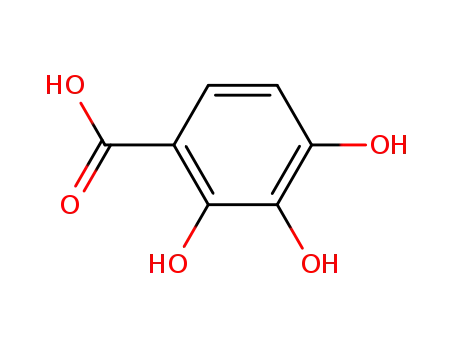
2,3,4-trihydroxybenzoic acid
| Conditions | Yield |
|---|---|
|
|
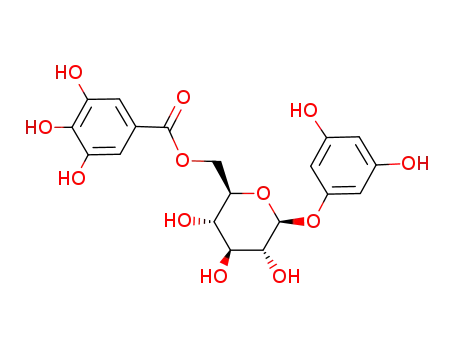
6-galloyl-1-O-(phloroglucinol)-glucopyranose


3,4,5-trihydroxybenzoic acid

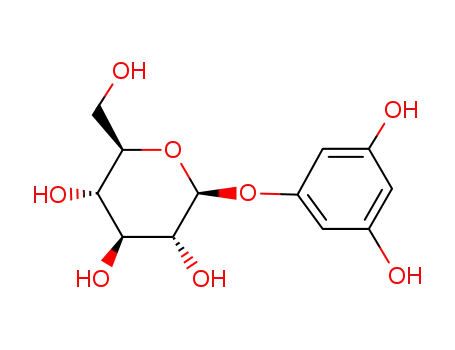
1-β-D-glucopyranosyloxy-3,5-dihydroxybenzene
| Conditions | Yield |
|---|---|
|
With
tannase; water;
for 0.5h;
Ambient temperature;
|
3 mg |
|
With
tannase; water;
for 0.5h;
Ambient temperature;
|
|
|
With
tannase; water;
for 0.5h;
Product distribution;
|
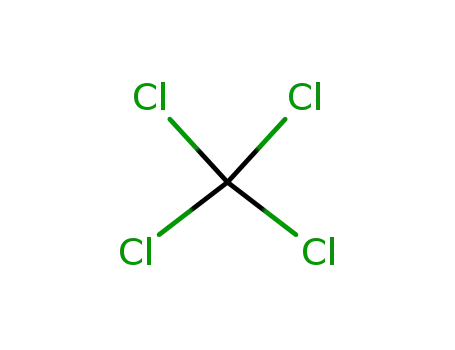
tetrachloromethane
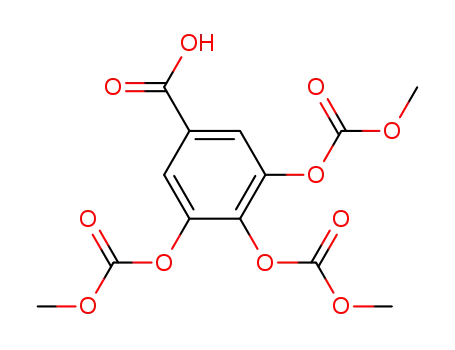
3,4,5-tris[(methoxycarbonyl)oxy]benzoic acid
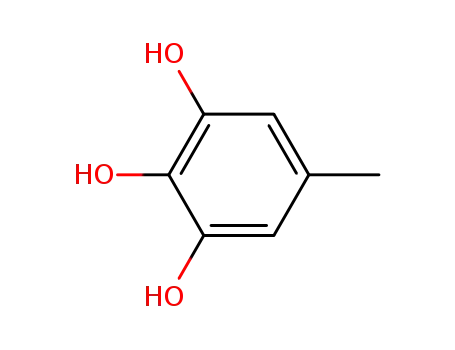
5-methylpyrogallol
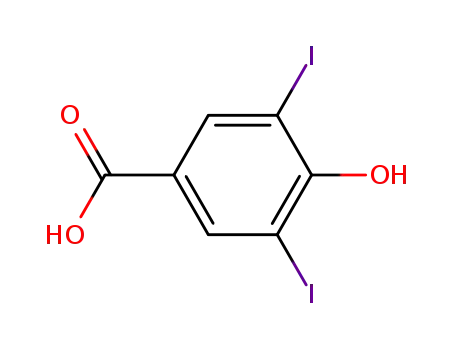
3,5-diiodo-4-hydroxybenzoic acid
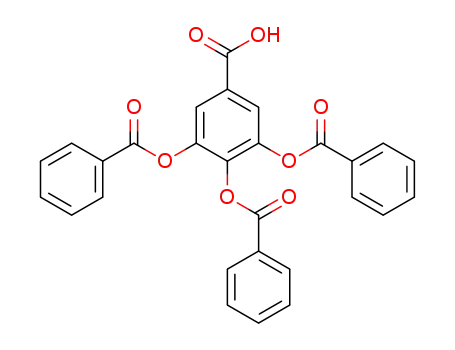
3,4,5-tris-benzoyloxy-benzoic acid
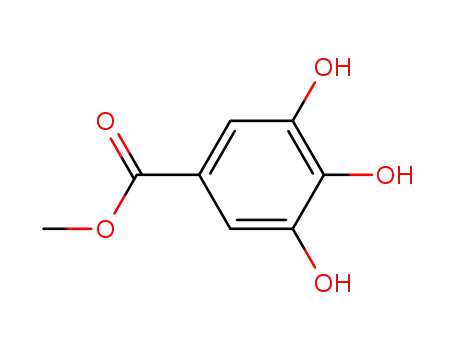
methyl galloate
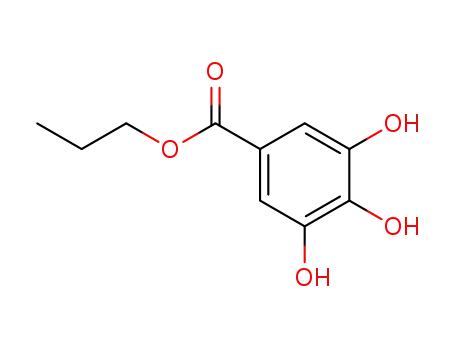
Propyl gallate
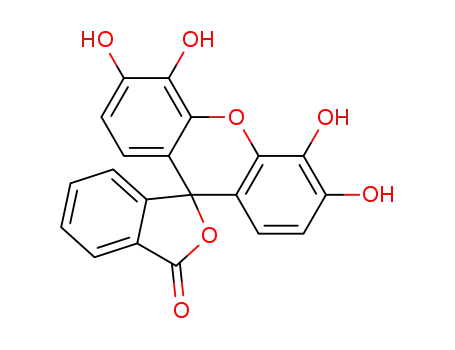
gallein