Your Location:Home > Products > Herbs Extract and Health Care > Propyl Gallate
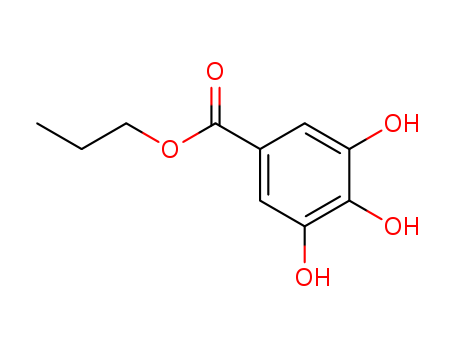


CasNo: 121-79-9
MF: C10H12O5
Appearance: white to light beige crystalline powder
|
Preparation |
Produced commercially by the esterification of gallic acid with propyl alcohol followed by distillation to remove the excess alcohol. |
|
Production Methods |
Propyl gallate is prepared by the esterification of 3,4,5-trihydroxybenzoic acid (gallic acid) with n-propanol. Other alkyl gallates are prepared similarly using an appropriate alcohol of the desired alkyl chain length. |
|
General Description |
Fine white to creamy-white crystalline powder. Odorless or with a faint odor. Melting point 150°C. Insoluble in water. Slightly bitter taste. |
|
Air & Water Reactions |
Insoluble in water. |
|
Reactivity Profile |
Propyl gallate can react with oxidizing agents. Incompatible with strong acids, strong bases and strong reducing agents. Darkens in the presence of iron and iron salts. Contact with metals should be avoided . |
|
Hazard |
Use in foods restricted to 0.02% of fat con- tent. |
|
Fire Hazard |
Propyl gallate is combustible. |
|
Flammability and Explosibility |
Notclassified |
|
Pharmaceutical Applications |
Propyl gallate has become widely used as an antioxidant in cosmetics, perfumes, foods, and pharmaceuticals since its use in preventing autoxidation of oils was first described in 1943.It is primarily used, in concentrations up to 0.1% w/v, to prevent the rancidity of oils and fats;it may also be used at concentrations of 0.002% w/v to prevent peroxide formation in ether, and at 0.01% w/v to prevent the oxidation of paraldehyde. Synergistic effects with other antioxidants such as butylated hydroxyanisole and butylated hydroxytoluene have been reported. Propyl gallate is also said to possess some antimicrobial properties; Studies have shown that, when added to powder blends containing ketorolac, propyl gallate significantly increases the drug stability in the preparation. Other alkyl gallates are also used as antioxidants and have approximately equivalent antioxidant properties when used in equimolar concentration; however, solubilities vary; Propyl gallate has also been investigated for its therapeutic properties, mainly in animal studies. |
|
Contact allergens |
This gallate ester (E 311) is an antioxidant frequently used in the food, cosmetic, and pharmaceutical industries to prevent the oxidation of unsaturated fatty acids into rancid-smelling compounds. It causes cosmetic dermatitis mainly from lipsticks and induced contact dermatitis in a baker, and in a female confectioner, primarily sensitized by her night cream, who fried doughnuts the margarine probably containing gallates. |
|
Biochem/physiol Actions |
An antioxidant that exhibits antimicrobial activity. Propyl gallate has been reported to be an effective antioxidant-based hepatoprotector, both in vitro and in vivo. It has also been shown to prevent neuronal apoptosis and block the death of neurons exposed to FeSO4/GA as well as partially protect endothelial cells against TNF-induced apoptosis. |
|
Toxicology |
Propyl gallate (n-propyl-3,4,5-trihydroxybenzoate) is used in vegetable oils and butter. When 1.2 or 2.3% propyl gallate was added to feed for rats, loss of weight was observed. This may be due to the rats reluctance to eat food that was contaminated with the bitter taste of propyl gallate. When it was given for 10 to 16 months at the 2 to 3% level, 40% of the rats died within the first month and the remainder showed severe growth inhibition. Autopsies of rats indicated kidney damage resulting from the ingestion of propyl gallate. However, no other animal studies show serious problems and further studies indicated that propyl gallate does not cause serious chronic toxicities. |
|
Safety Profile |
Poison by ingestion and intraperitoneal routes. Experimental teratogenic and reproductive effects. Questionable carcinogen with experimental tumorigenic data. Mutation data reported. Combustible when exposed to heat or flame; can react with oxidizing materials. When heated to decomposition it emits acrid smoke and irritating fumes. |
|
Safety |
It has been reported, following animal studies, that propyl gallate has a strong contact sensitization potential.Propyl gallate has also produced cytogenic effects in CHO-K1 cells.However, despite this, there have been few reports of adverse reactions to propyl gallate.Those that have been described include contact dermatitis, allergic contact dermatitis,and methemoglobinemia in neonates. The WHO has set an estimated acceptable daily intake for propyl gallate at up to 1.4 mg/kg body-weight. (cat, oral): 0.4 g/kg (mouse, oral): 1.7 g/kg (rat, oral): 2.1 g/kg (rat, IP): 0.38 g/kg |
|
storage |
Propyl gallate is unstable at high temperatures and is rapidly destroyed in oils that are used for frying purposes. The bulk material should be stored in a well-closed, nonmetallic container, protected from light, in a cool, dry place. |
|
Purification Methods |
Crystallise the ester from aqueous EtOH or *C6H6 (m 146-146.5o). [Beilstein 10 III 2078, 10 IV 2003.] |
|
Incompatibilities |
The alkyl gallates are incompatible with metals, e.g. sodium, potassium, and iron, forming intensely colored complexes. Complex formation may be prevented, under some circumstances, by the addition of a sequestering agent, typically citric acid. Propyl gallate may also react with oxidizing materials. |
|
Regulatory Status |
GRAS listed. Accepted for use as a food additive in Europe. Included in the FDA Inactive Ingredients Database (IM injections; oral, and topical preparations). Included in nonparenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients. |
InChI:InChI:1S/C10H12O5/c1-2-3-15-10(14)6-4-7(11)9(13)8(12)5-6/h4-5,11-13H,2-3H2,1H3
One of the tannase isoforms produced by ...
A bio-imprinting technique was applied t...
Improvement of transesterification-catal...
The conformational stability of enzyme h...
The development of cucurbit[n]urils-poly...
Equilibrium measurements at several temp...
Improving the catalytic capability of en...
Enzymatic synthesis of propyl gallate in...
In the isolated rat thoracic aorta, gall...
The ability of a non-commercial immobili...
Immobilised derivatives of tannase from ...
The esterification reaction used to prep...
With more than 200 million cases and 400...
The present invention provides a kind of...
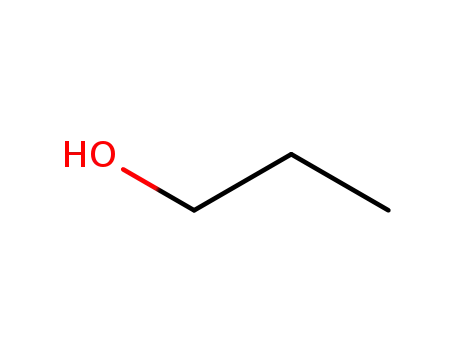
propan-1-ol

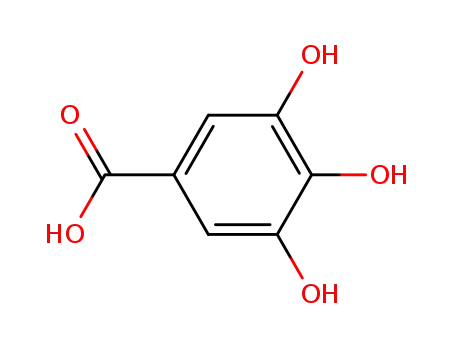
3,4,5-trihydroxybenzoic acid

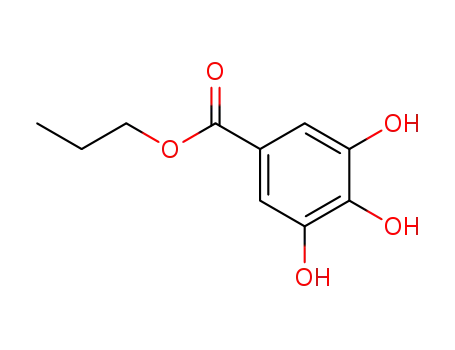
Propyl gallate
| Conditions | Yield |
|---|---|
|
With
brominated modified sulfonic acid resin;
at 100 ℃;
for 5h;
|
98% |
|
With
toluene-4-sulfonic acid;
at 107 ℃;
for 0.133333h;
under 9308.91 Torr;
Temperature;
Wavelength;
Sealed tube;
Microwave irradiation;
|
94% |
|
With
cucurbit[6]uril-supported Keggin-type silicotungstic acid Q[6]-STA;
at 96.84 ℃;
for 4h;
Time;
Reagent/catalyst;
Catalytic behavior;
|
94.7% |
|
With
thionyl chloride;
at 35 - 65 ℃;
for 1h;
|
91.6% |
|
With
CaCO3-immobilized Staphylococcus xylosus lipase;
at 50 ℃;
Enzymatic reaction;
|
89.4% |
|
With
sulfuric acid;
for 6h;
Reflux;
|
86% |
|
With
perchloric acid;
In
benzene;
at 80 - 90 ℃;
Reagent/catalyst;
|
81.6% |
|
With
sulfuric acid;
In
toluene;
Heating;
|
74% |
|
With
thionyl chloride;
Inert atmosphere;
|
73% |
|
propan-1-ol; 3,4,5-trihydroxybenzoic acid;
With
diisopropyl-carbodiimide;
In
tetrahydrofuran;
at 0 ℃;
for 1h;
With
dmap;
In
tetrahydrofuran;
at 0 ℃;
for 6h;
|
66% |
|
With
tannase; water;
In
toluene;
at 20 - 35 ℃;
Equilibrium constant;
Thermodynamic data;
ΔrHmo, ΔrGmo, ΔrSmo, reaction time 6-7 d, other reagents: K2HPO4, H3PO4; CH3COONa, CH3COOH; NaOH, 2-(N-morpholino)ethanesulfonic acid; reaction in aq. soln. at various pH, other reaction time;
|
|
|
With
diethyl sulfate; benzene;
|
|
|
With
sulfuric acid monopropyl ester; benzene;
|
|
|
With
di-1-propyl sulfate; benzene;
|
|
|
With
hydrogenchloride;
|
|
|
With
sulfuric acid;
|
|
|
With
dicyclohexyl-carbodiimide;
In
tetrahydrofuran;
at 0 ℃;
for 20h;
|
|
|
With
acetyl chloride;
|
|
|
With
dicyclohexyl-carbodiimide;
In
1,4-dioxane;
at 5 ℃;
for 48h;
|
|
|
With
dicyclohexyl-carbodiimide;
In
1,4-dioxane;
at 5 ℃;
for 48h;
|
|
|
With
dicyclohexyl-carbodiimide;
In
1,4-dioxane;
at 5 ℃;
for 48h;
|
|
|
In
benzene;
at 30 ℃;
for 48h;
Enzymatic reaction;
|
|
|
With
dicyclohexyl-carbodiimide;
In
tetrahydrofuran; dichloromethane;
at 20 ℃;
|
|
|
With
citric acid;
at 60 ℃;
for 2h;
Temperature;
|
|
|
With
mordenite;
at 50 - 70 ℃;
for 5h;
Temperature;
Molecular sieve;
|
2.40 g |
|
With
thionyl chloride;
for 5.5h;
Reflux;
|
|
|
With
sulfuric acid;
Reflux;
|
|
|
With
thionyl chloride;
for 5.5h;
Reflux;
|
|
|
With
2C40H44N24O12*9H(1+)*(x)H2O*3Mo12O40P(3-);
at 80 ℃;
for 5h;
Time;
Catalytic behavior;
|

3,4,5-trihydroxybenzoic acid

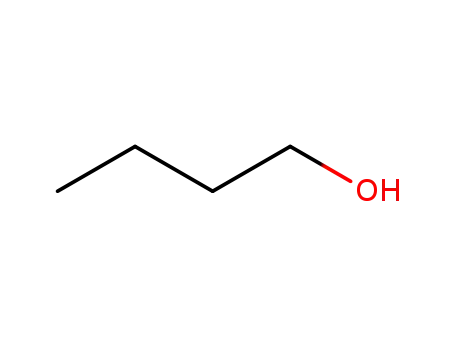
butan-1-ol


Propyl gallate
| Conditions | Yield |
|---|---|
|
With
sulfuric acid;
In
propan-1-ol; 2-methyl-propan-1-ol; water;
|
75% |

propan-1-ol

3,4,5-trihydroxybenzoic acid

butan-1-ol
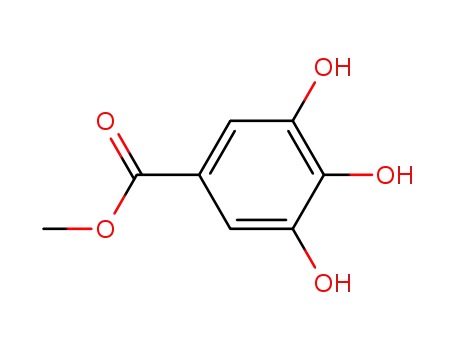
methyl galloate
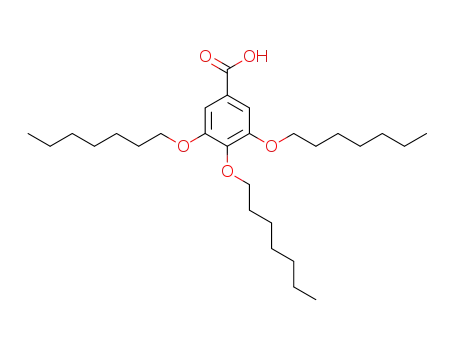
3,4,5-tris(heptyloxy)benzoic acid
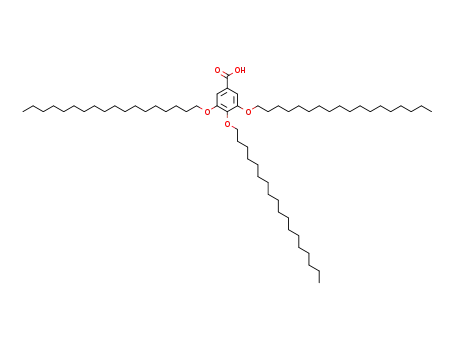
3,4,5-tris(n-octadecan-1-yloxy)benzoic acid

propan-1-ol

3,4,5-trihydroxybenzoic acid