Your Location:Home > Products > Food Ingredients > Ammonium Ferric Citrate
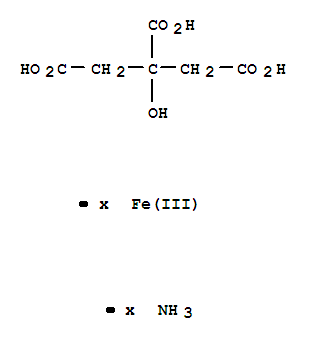
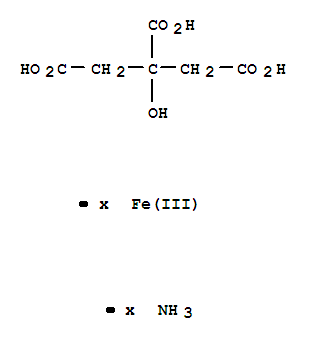
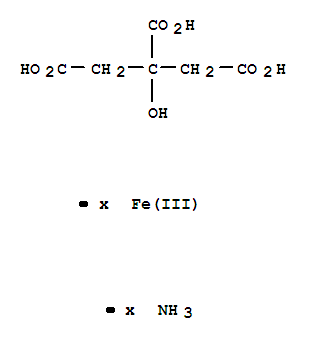
CasNo: 1185-57-5
MF: C6H8O7.xFe.xH3N
Appearance: Ferric ammonium citrate is a yellowish brown to red solid with a faint odor of ammonia.
|
Maximal allowable residue and maximal allowable usage amount |
Name of additive: Ammonium ferric citrate [Mineral] Function of additive: Nutrition Enhancer ?Maximal allowable residue(g/kg): / ▼▲ Food allowed to use it as additive Maximal allowable amount(g/kg) Salt, sandwich sugar 4000~8000mg (1: strengthened amount calculated on the element iron: cereal and its products 24~48mg/kg; beverage:10~20mg/kg dairy products; infant food 60~100mg/kg; sandwich sugar 600~1200 mg; 2. the iron content in various kinds of salts: ferrous sulfate (contains seven crystal water molecules), ?ferrous lactate (Including three crystal water molecules) 19.39%; iron citrate (containing 5 crystal water molecules ) 16.67%; ferrous fumarate:32.9%; ferrous gluconic acid:12%; ferric ammonium citrate 16% 3. Iron source can also be applied of the heme iron extracted from the pig blood with calculation on the iron elements upon strengthening 4. Other iron salts such as ferrous carbonate, ferrous citrate, ferrous fumarate, ferrous succinate, reduced iron, electrolytic iron can also apply it; calculated on the element iron upon strengthening. High-iron cereals and their products (daily limit of such food: 50g) ? 1200~1350mg (1 the strengthening amount is calculated on the element iron: cereals and their products: 24~48mg/kg; drinks 10~20mg/kg; dairy products, infant food: 60~100mg/kg; sandwich sugar: 600~1200mg 2. The iron content in various kinds of iron salts ferrous sulfate (contains seven crystal water molecules), ?ferrous lactate (Including three crystal water molecules) 19.39%; iron citrate (containing 5 crystal water molecules ) 16.67%; ferrous fumarate:32.9%; ferrous gluconic acid:12%; ferric ammonium citrate 16% 3. Iron source can also be applied of the heme iron extracted from the pig blood with calculation on the iron elements upon strengthening 4. Other iron salts such as ferrous carbonate, ferrous citrate, ferrous fumarate, ferrous succinate, reduced iron, electrolytic iron can also apply it; calculated on the element iron upon strengthening. ? ? Dairy products, infant food 400~800mg (1 the strengthening amount is calculated on the element iron: cereals and their products: 24~48mg/kg; drinks 10~20mg/kg; dairy products, infant food: 60~100mg/kg; sandwich sugar: 600~1200mg 2. The iron content in various kinds of iron salts ferrous sulfate (contains seven crystal water molecules), ?ferrous lactate (Including three crystal water molecules) 19.39%; iron citrate (containing 5 crystal water molecules ) 16.67%; ferrous fumarate:32.9%; ferrous gluconic acid:12%; ferric ammonium citrate 16% 3. Iron source can also be applied of the heme iron extracted from the pig blood with calculation on the iron elements upon strengthening 4. Other iron salts such as ferrous carbonate, ferrous citrate, ferrous fumarate, ferrous succinate, reduced iron, electrolytic iron can also apply it; calculated on the element iron upon strengthening. Drink 70~140mg (1 the strengthening amount is calculated on the element iron: cereals and their products: 24~48mg/kg; drinks 10~20mg/kg; dairy products, infant food: 60~100mg/kg; sandwich sugar: 600~1200mg 2. The iron content in various kinds of iron salts ferrous sulfate (contains seven crystal water molecules), ?ferrous lactate (Including three crystal water molecules) 19.39%; iron citrate (containing 5 crystal water molecules ) 16.67%; ferrous fumarate:32.9%; ferrous gluconic acid:12%; ferric ammonium citrate 16% 3. Iron source can also be applied of the heme iron extracted from the pig blood with calculation on the iron elements upon strengthening 4. Other iron salts such as ferrous carbonate, ferrous citrate, ferrous fumarate, ferrous succinate, reduced iron, electrolytic iron can also apply it; calculated on the element iron upon strengthening. ? Cereals and their products 160~330mg (1 the strengthening amount is calculated on the element iron: cereals and their products: 24~48mg/kg; drinks 10~20mg/kg; dairy products, infant food: 60~100mg/kg; sandwich sugar: 600~1200mg 2. The iron content in various kinds of iron salts ferrous sulfate (contains seven crystal water molecules), ?ferrous lactate (Including three crystal water molecules) 19.39%; iron citrate (containing 5 crystal water molecules ) 16.67%; ferrous fumarate:32.9%; ferrous gluconic acid:12%; ferric ammonium citrate 16% 3. Iron source can also be applied of the heme iron extracted from the pig blood with calculation on the iron elements upon strengthening 4. Other iron salts such as ferrous carbonate, ferrous citrate, ferrous fumarate, ferrous succinate, reduced iron, electrolytic iron can also apply it; calculated on the element iron upon strengthening. |
|
Production method |
The ferric hydroxide is dissolved in citric acid, neutralized with ammonia solution, followed by drying to obtain it. (1) Preparation of ferric hydroxide Ferrous sulfate was added to the water, and sulfuric acid was slowly added thereto under stirring, followed by addition of an aqueous sodium chlorate solution. Then stir vigorously and raise the temperature to above 80 ℃; further add sodium chlorate. Stir until the reaction is terminated (no further ferrous reaction through ferricyanide test) to obtain the ferric sulfate solution. Add this solution to the reaction tank, add sodium hydroxide solution and stir vigorously with the reaction temperature of 80-90 °; when the reaction solution turns from viscous to thin, add water for washing until the sulfate and chlorine meet the requirements. Dry to get the iron hydroxide. (2) Preparation of ferric ammonium citrate; add citrate, ferric hydroxide and water into the reaction tank, stir and control the temperature at 95 ℃ above and maintain the temperature for 1h. And then cooled to 50?C and sent into ammonia under stirring. Stand for more than 48h. The supernatant was filtered and the filtrate was concentrated to a paste and dried at 80 ° C to obtain ferric ammonium citrate. The total yield of ferrous sulfate is 73-75%. The same procedure as in preparation method 2 of brown ferric ammonium citrate. Adding ammonium hydroxide to the ferric sulfate to generate ferrous hydroxide precipitate; filter and wash with water to no sulfate reaction anymore; then add? citrate and heat to 60?C to completely dissolve the precipitate; neutralize with ammonia hydroxide and concentrate to a syrupy state, and coat on a glass plate, and dry to obtain a small sheet product. Fe2 (SO4) 3 + 6NH3 + 6H2O → 3 (NH4) 2SO4 + 2Fe (OH) 3 ↓ 2C6H8O7 + 3NH4OH + Fe (OH) 3 → (C6H5O7) 2Fe (NH4) 3 + 6H2O It can also taken of ferrous sulfate as raw materials. 28g FeSO4 ? 7H2O was dissolved in 4 mL water; add 3 mL 98% concentrated sulfuric acid and then drop 5mL 36% hydrogen peroxide; heat to 83 ℃ and stir for 0.5h; the blue color upon potassium ferricyanide test can tell the termination of the reaction; further add 100 mL distilled water, and 40% NaOH was added at 80 ° C with stirring to make the solution be alkaline. The precipitate was suction filtered and washed with hot water until no sulfate was present any more. The prepared Fe (OH) 3 was added to a citric acid solution (14 g/100 mL water) and stirred at 95 ° C for 1 h. The solution was then cooled to 50 ° C and an appropriate amount of aqueous ammonia was added. The solution was cooled to room temperature. Evaporate the supernatant to a paste with drying at 60~80 ° C to obtain the product with a yield of 91%. A considerable amount of citric acid solution was added to ferric hydroxide produced from reaction between the ferric sulfate and aqueous ammonia; the concentrated slurry was coated on a glass plate, dried, and then peeled off from the glass plate. Ferric hydroxide is dissolved in citric acid, with neutralization with ammonia hydroxide and evaporation at 60 ° C to obtain. |
|
Hazards & Safety Information |
Category:? Toxic substances Toxic classification:?? poisoning Acute toxicity :? Oral-rat LD:> 2000 mg/kg Flammable and hazardous characteristics:?? being combustible with fire discharging iron and nitrogen oxide-containing spicy smoke Storage and transport characteristics:?? Treasury: low temperature, ventilated, dry Fire extinguishing agent :? water, carbon dioxide, dry powder, sand Occupational Standard:?? TWA 1 mg/m3 |
|
General Description |
Ammonium ferric citrate is a yellowish brown to red solid with a faint odor of ammonia. Ammonium ferric citrate is soluble in water. The primary hazard is the threat to the environment. Immediate steps should be taken to limit its spread to the environment. Ammonium ferric citrate is used in medicine, in making blueprints, and as a feed additive. |
|
Air & Water Reactions |
Water soluble. |
|
Reactivity Profile |
Acidic salts, such as Ammonium ferric citrate, are generally soluble in water. The resulting solutions contain moderate concentrations of hydrogen ions and have pH's of less than 7.0. They react as acids to neutralize bases. These neutralizations generate heat, but less or far less than is generated by neutralization of inorganic acids, inorganic oxoacids, and carboxylic acid. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible. Many of these compounds catalyze organic reactions. Special Hazards of Combustion Products: Toxic oxides of nitrogen or ammonia gas may be formed in fires [USCG, 1999]. |
|
Health Hazard |
Inhalation of dust irritates nose and throat. Ingestion causes irritation of mouth and stomach. Dust irritates eyes and causes mild irritation of skin on prolonged contact. |
|
Fire Hazard |
Special Hazards of Combustion Products: Toxic oxides of nitrogen or ammonia gas may be formed in fires. |
|
Flammability and Explosibility |
Notclassified |
|
Potential Exposure |
Ferric ammonium citrate is used in blueprinting, photography, medical treatment; and as an animal food additive. |
|
Shipping |
UN3077 Environmentally hazardous substances, solid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required. |
|
Incompatibilities |
Compounds of the carboxyl group react with all bases, both inorganic and organic (i.e., amines) releasing substantial heat, water and a salt that may be harmful. Incompatible with arsenic compounds (releases hydrogen cyanide gas), diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides (releasing heat, toxic and possibly flammable gases), thiosulfates and dithionites (releasing hydrogen sulfate and oxides of sulfur). |
InChI:InChI=1/C7H10O7.Fe.H3N/c8-4(9)1-7(14,2-5(10)11)3-6(12)13;;/h14H,1-3H2,(H,8,9)(H,10,11)(H,12,13);;1H3