Your Location:Home > Products > Food Ingredients > D-Psicose


CasNo: 551-68-8
MF: C6H12O6
Appearance: Sweet syrupy liquid
|
Enzyme inhibitor |
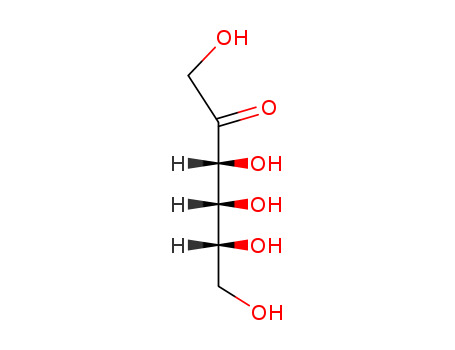
This ketohexose (FW = 180.16 g/mol), also known as D-ribo-2-hexulose and reportedly fpound in cane molasses, has epimers of D-psicose are Dfructose, D-sorbose, and L-tagatose. D-Psicose is an alternative substrate for fructokinase. The structural composition of D-psicose at 27°C in D2O is 22% a-pyranose, 24% b-pyranose, 39% a-furanose, and 15% bfuranose. Target(s): ketohexokinase, or hepatic fructokinase; also alternative substrate. |
|
Definition |
ChEBI: The D-enantiomer of psicose. |
InChI:InChI=1/C6H12O6/c7-1-3(9)5(11)6(12)4(10)2-8/h3,5-9,11-12H,1-2H2/t3-,5-,6+/m1/s1
Synthesis of a pentasil-type zeolite wit...
(NH4)5H6PV8Mo4O40 supported on hydroxyap...
Processes for converting glucose to sorb...
Bio-refinery is attracting significant i...
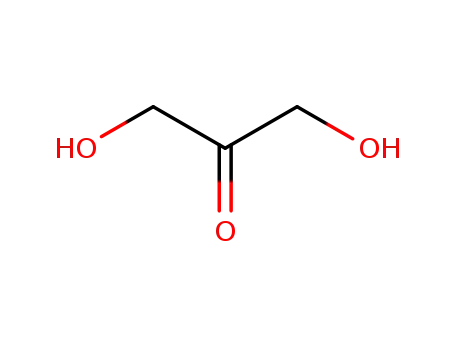
dihydroxyacetone

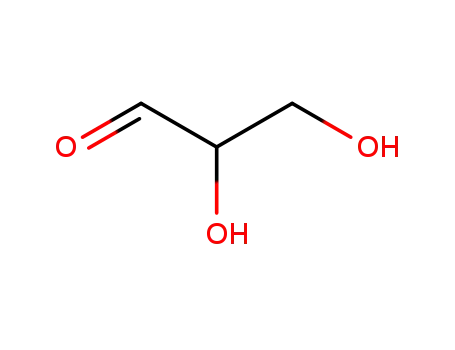
Glyceraldehyde

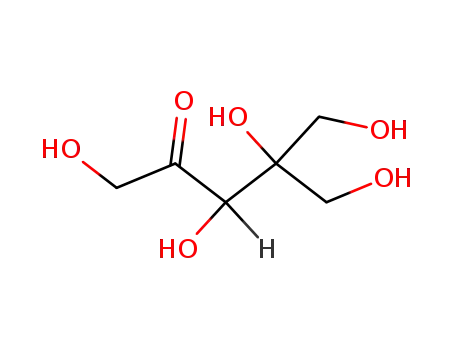
DL-dendroketose

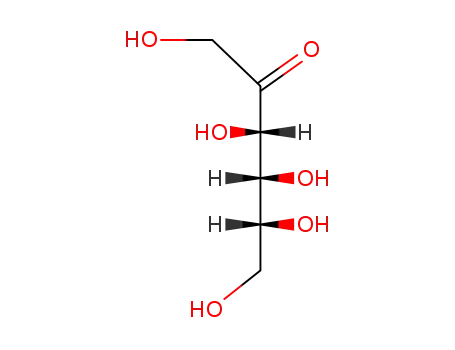
fructose

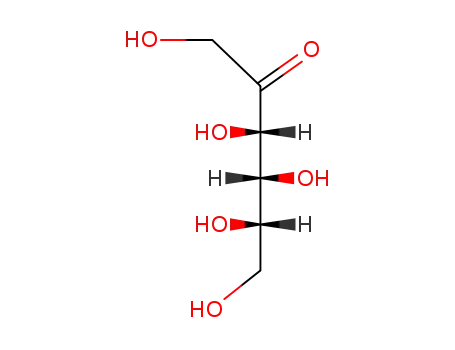
sorbose

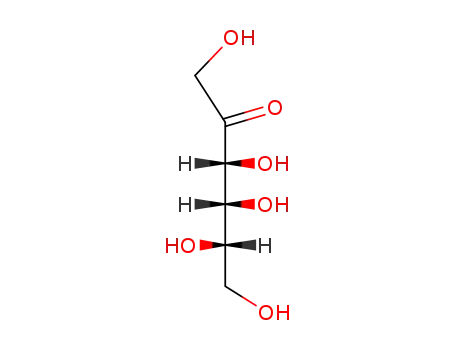
lyxo-2-hexulose

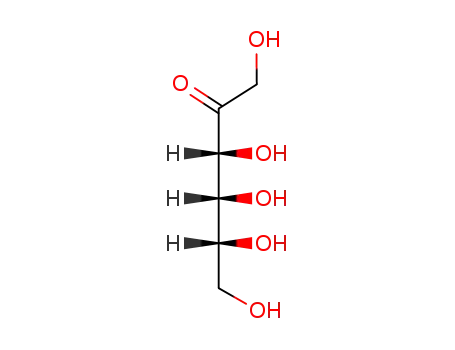
ribo-2-hexulose
| Conditions | Yield |
|---|---|
|
sodium hydroxide;
In
water;
at 25 ℃;
for 1h;
Product distribution;
further catalysts;
|
8 % Chromat. 2 % Chromat. 40 % Chromat. 32 % Chromat. 18 % Chromat. |

dihydroxyacetone


Glyceraldehyde


DL-dendroketose


fructose


sorbose


lyxo-2-hexulose


ribo-2-hexulose
| Conditions | Yield |
|---|---|
|
sodium hydroxide;
In
water;
at 25 ℃;
for 1h;
Product distribution;
further catalysts;
|
8 % Chromat. 2 % Chromat. 40 % Chromat. 32 % Chromat. 18 % Chromat. |
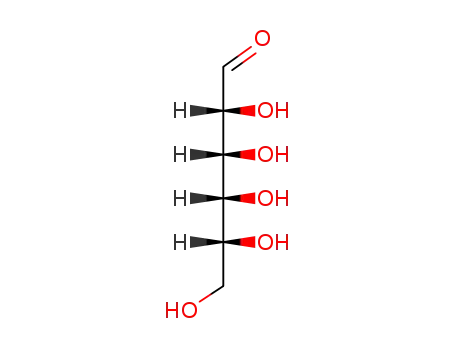
D-glucose
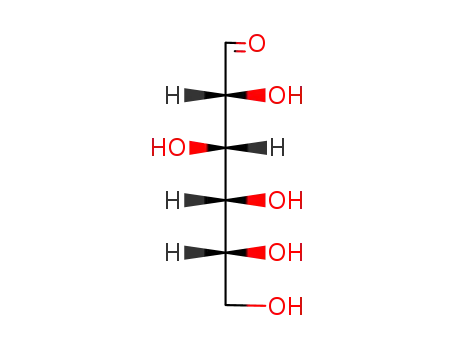
D-Allose
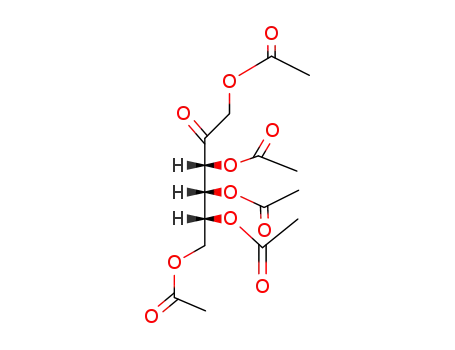
Penta-O-acetyl-keto-D-psicose
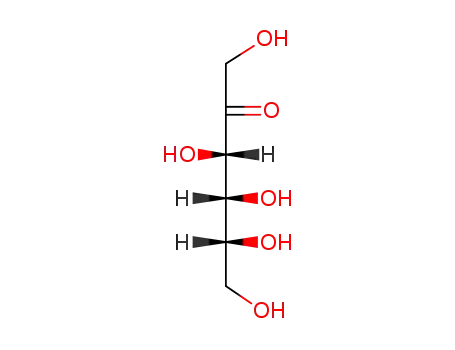
D-Fructose
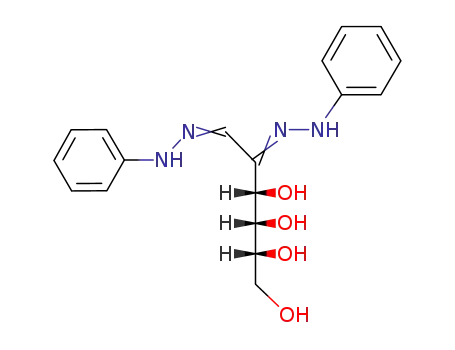
D-allose osazone
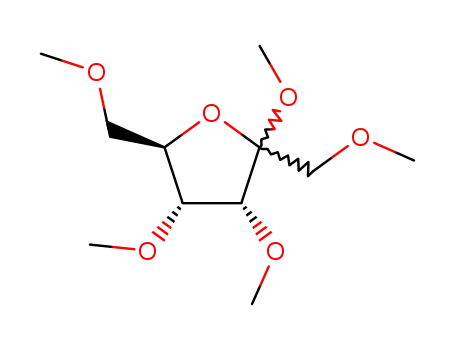
methyl-[tetra-O-methyl-psicofuranoside
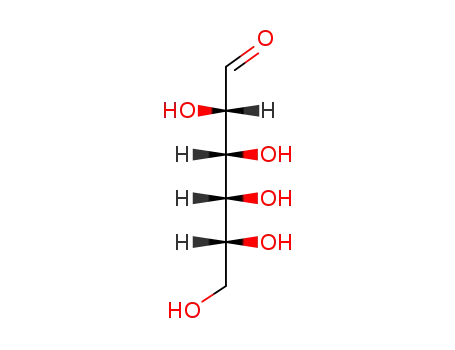
D-altrose

D-Allose