Your Location:Home > Products > Herbs Extract and Health Care > Hesperidin


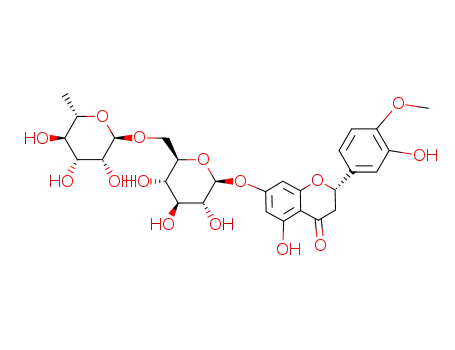
CasNo: 520-26-3
MF: C28H34O15
Appearance: light brown powder
|
Hesperidin |
Hesperidin (glycoside) is a flavonoid substance which has flavanone oxygen glycoside structure. It is weakly acidic with pure being white needle-like crystals. It is also the main component of vitamin P. Figure 1 the molecular structure of hesperidin. After the hydrogenation, hesperidin becomes a natural sweetener, dihydrochalcone, whose sweetness is 1,000 times as high as sucrose. It can be used as a functional food for application. Hesperidin has a variety of biological characteristics. Modern research has found that: hesperidin has various effects such as antioxidant, anti-cancer, anti-mildew, anti-allergy, lowering blood pressure, inhibiting oral cancer and esophageal cancer, maintenance of osmotic pressure, increasing capillary toughness, and lowering cholesterol. Related studies have shown that hesperidin has broad antibacterial spectrum on common food contamination. It has significant inhibitory effect on Bacillus subtilis, Salmonella typhimurium, Shigella bacteria, hemolytic streptococcus and Vibrio cholerae. Therefore, it is widely applied in food additives and food processing. |
|
Pharmacological activity |
1. Hesperidin is a kind of drug for treatment of hypertension and myocardial infarction. It is used as the pharmaceutical raw material in the pharmaceutical industry and is one of the main components of a Chinese patent medicine, beniol. 2. Hesperidin has various effects such as anti-lipid oxidation, scavenging oxygen free radicals, and anti-inflammatory, anti-viral, anti-bacterial. Long-term use can delay aging and cancer. In short, hesperidin is a kind of flavonoids with clear defined pharmacological activity as well as extensive function of flavonoids. In addition to its application in medicine, it also has wide application in sports pharmacy and sports nutrition and therefore has broad prospects of development and utilization. Its related research work is expected to subject to further deepening. |
|
Industry Status |
Hesperidin has various effects such as maintaining osmotic pressure, increasing capillary toughness, shortening the bleeding time, and reducing the cholesterol. It is clinically used for the adjuvant treatment of cardiovascular diseases. It can be used for cultivating various kinds of drugs for preventing arteriosclerosis and myocardial infarction. It is one of the major raw materials for synthesizing Chinese patent medicine “beniol”. It can be used as natural antioxidants in the food industry and can also be used in the cosmetics industry. Hesperidin is mostly presented in the waste of citrus processing such as fruit skin and fruit bag with the highest content being presented in mature skin and tissue (30%-50% in the peel, orange envelope, nuclear, pulp contains 30%-50%, epicarp contains 10%-20%). Orange juice and capsule contain a relative low amount being 1% to 5%. Extraction methods of hesperidin include solvent extraction, alkaline extraction and acid precipitation, carbon adsorption, ion exchange, wherein the alkali extraction and acid precipitation method is simple, low-cost, and has a high extraction rate. Hesperidin can be dissolved in dilute alkali and pyridine as well as hot water (over 70 °C). It is also slightly soluble in methanol but almost insoluble in acetone, benzene and chloroform. The extraction of hesperidin mainly take advantage of its two phenolic hydroxyl groups which under alkaline conditions, has reaction with the sodium ion in the solution to generate sodium salt to be dissolved out; then acidify, cool to precipitate it from solution. Extraction of hesperidin from citrus peels commonly adopts heat extraction and soaks extraction method with a non-idea yield. In recent years, studies on the ultrasonic extraction of the effective components from natural plants (especially herbs) have been widely carried out, and have already obtained some progress. The extraction is first based on the hesperidins’ ring-opening dissolution under alkaline conditions and then further loop closure precipitation for being separated out under acidic conditions. During the extraction process, increasing the amount of the alkali can reduce the necessary amount of ethanol. But it is not recommended to apply a relative large amount of alkaline otherwise hesperidin is easily susceptible to oxidation damage. Figure 2 Yellow powder of hesperidin The above information is edited by the lookchem of Dai Xiongfeng. |
|
Production method |
The product is presented in the pericarp of lemon, citrus, and Citus aurantium. In citrus, the developed system of the mesocarp (white spongy tissue) mostly contains citrus glycosides.。 Instead thinner system of mesocarp mostly contains hesperidin. The product is mainly extracted from the dried, ripe orange peel. Crush the dry orange peel; add 3-6 times the amount of water to soak for about 0.5h to make it soft. Then add 4-10% of the amount of lime and 7-12 times the amount of water; stir uniformly and check the pH. The pH value should reach 11.5-12, otherwise we should supplement lime or sodium hydroxide. After soaking for 1.5-2h, centrifuge and filter with the residues adding 5-7 times the amount of water and further adjust to pH 11.5-12 with proper amount of lime; continue soaking and centrifuge and filter again. After the clarification of the filtrate, add diluted hydrochloric acid for adjusting pH to 5; stand for 2d; collect the precipitate and wash with water to nearly neutral which give the crude product. Add 1% of sodium hydroxide and 50% of ethanol to dissolve the crude product; filter and adjust the filtrate to pH 5 with dilute hydrochloric acid, stand overnight, and collect the precipitate; first wash once with 50% ethanol, and then wash with water to nearly neutral; dry at 70 °C; pulverize and sieve to obtain hesperidin with the total yield being 0.6-1.8%. |
|
History |
Hesperidin is the glycoside in the form of hesperidin and rubiose and is a derivative of dihydroflavonoids. It widely exists in legume, birch, lip flower, butterfly flower, Rutaceae, and citrus plants. Hesperidin is an important composition of citrus pulp and peel; most of hesperidin exists in citrus processing waste such as skin and fruit bag. Mature skin and tissue have the highest content of hesperidin (30–50% in endocarp; 30–50% in orange collaterals, nuclear, and pulp; and 10–20% in exo_x005fcarp); the content of hesperidin is relatively low in juice and orange bag, which is about 1–5%. The crude extracts of hesperidin was first discovered in 1827 by Lebreton. Then the Hungarian scholar Albert Szent-Gyorgi discovered that the flavonoids have a protective microvascular effect in 1936, which is similar to that of vitamin P. Preparation of vitamin P was made in 1938. It was not until 1949 that it was discovered that vitamin P was made up of two flavonoids, luteolin and hesperidin, which are believed to be vitamin active. This substance, which was later named as vitamin P, was designed to reduce blood vessel permeability and brittleness, as well as alleviate bad blood and vitamin C deficiency. It was later discovered that the substance had an antioxidant effect, so the name of vitamin P was abandoned. Due to the widespread distribution of hesperidin in plant medicine, the research and development have been widely followed. |
|
Indications |
Hesperidin can be used for cardiovascular disease prevention and treatment, blood sugar and blood lipid and blood pressure regulation, circulatory system regulation, and body regulation, and it can also be used as an antibacterial, anti-inflammatory, and antiviral. |
|
Flammability and Explosibility |
Nonflammable |
|
Pharmacology |
The pharmacological effect of hesperidin is widespread, and people thought it was vitamin P in the early days, but in recent years, people found that it has other functions such as controlling blood pressure, antiallergic, reducing bone mineral density and cholesterol, improving enzyme activity and microcirculation, antibacterial, anti-inflammatory, anti-hepatitis B, antitumor, and other pharmacological effects.Hesperidin has the function of vitamin P, which can reduce capillary permeability and prevent microvascular hemorrhage. Intraperitoneal injection of hesperidin at 175–250?mg/kg in mice could increase permeability of blood vessels by antihistamine and inhibiting hemolytic lecithin. Hesperidin has antiviral and antimicrobial effect, and preincubation with hesperidin at 200? mg/ml protects the cells from viruses. One to 10?μg/ml of hesperidin effectively inhibits the growth of the fungus. It has the effect of maintaining the normal osmotic pressure of the blood vessels, reducing the shortness of blood vessels, shortening bleeding time, reducing blood fat, and preventing atherosclerosis; hesperidin has an effect on the gastrointestinal tract, which can excite the smooth muscle transiently and then inhibit it, and it is a major component of the diet drug; hesperidin has an effect of anti-lipid peroxidation and scavenging hydroxyl radical. Hesperidin is a newly discovered flavonoid compound which has an effect in the central nervous system; it has a sedative effect. At the same time, hesperidin has the effect of lowering cholesterol, curing rheumatism, and inhibiting skin pigmentation. Hesperidin is a strong affinity for estrogen receptors, which can be used in estrogen receptors to prevent bone loss and reduce the number of osteoblasts. Hesperidin has a significant inhibitory effect on human lung cancer, colorectal cancer, kidney cancer, and human breast cancer cells, which can be used for cancer prevention. |
|
Purification Methods |
Dissolve hesperidine in dilute aqueous alkali and precipitate it by adjusting the pH to 6-7. [Beilstein 18 III/IV 3219, 18/5 V 218.] |
|
Physical properties |
Appearance: fine dendritic crystal (precipitation at pH 6–7), odorless, tasteless. Melting point: 258–262?°C (softening at 250?°C). Solubility: 1?g of hesperidin can be soluble in 50?l of water. Hesperidin dissolves in dimethyl formamide and formamide at 60?°C.?It is slightly soluble in methanol and hot ice acetic acid and hardly soluble in acetone, benzene, and chloroform and is soluble in dilute alkali and pyridine. |
|
Definition |
ChEBI: A disaccharide derivative that consists of hesperetin substituted by a 6-O-(alpha-L-rhamnopyranosyl)-beta-D-glucopyranosyl moiety at position 7 via a glycosidic linkage. |
InChI:InChI=1/C28H34O15/c1-10-21(32)23(34)25(36)27(40-10)39-9-19-22(33)24(35)26(37)28(43-19)41-12-6-14(30)20-15(31)8-17(42-18(20)7-12)11-3-4-16(38-2)13(29)5-11/h3-7,10,17,19,21-30,32-37H,8-9H2,1-2H3/t10?,17-,19?,21-,22+,23-,24-,25?,26?,27+,28+/m0/s1
A triazole-bridged bis(β-cyclodextrin) w...
Two flavanone glycosides were isolated f...
Glucosyl hesperidin (G-hesperidin) is a ...
Model phenoxyl and more complex flavonoi...
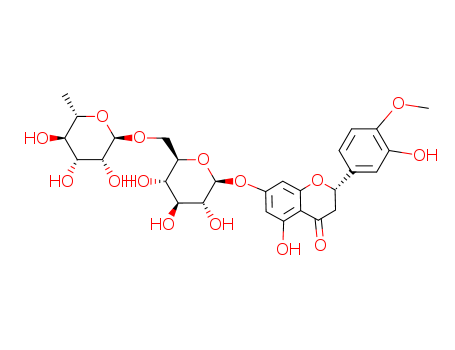
hesperidin

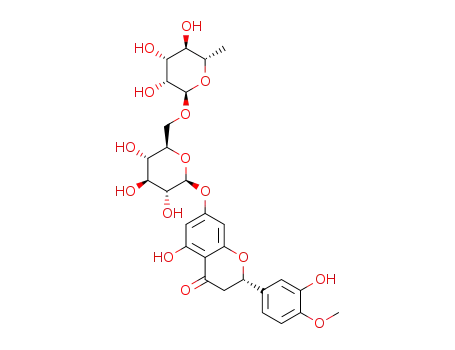
(R)-hesperidin

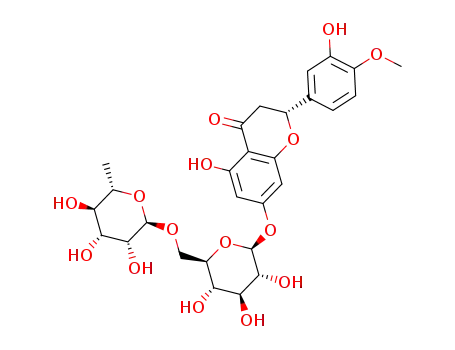
hesperidin
| Conditions | Yield |
|---|---|
|
In
methanol; formic acid; acetonitrile;
at 20 ℃;
Resolution of racemate;
|
4G-α-D-glucopyranosyl hesperidin


hesperidin
| Conditions | Yield |
|---|---|
|
With
small intestine homogenate of rat;
at 37 ℃;
for 1h;
|
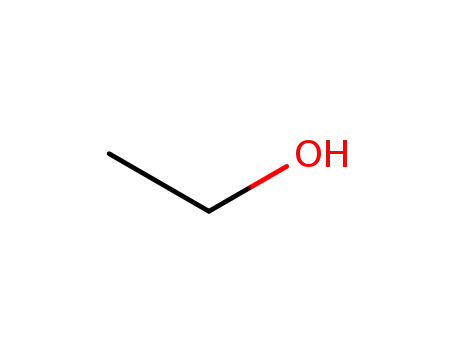
ethanol
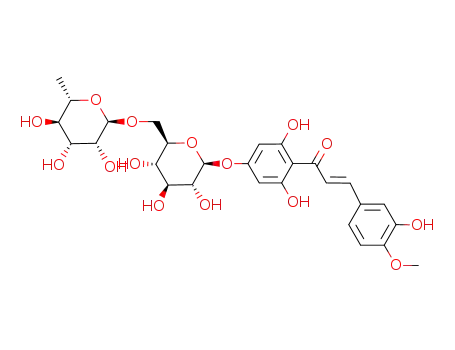
3,2',6'-trihydroxy-4-methoxy-4'-(O6-α-L-rhamnopyranosyl-β-D-glucopyranosyloxy)-trans-chalcone
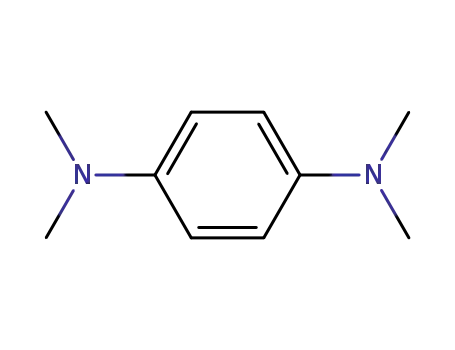
N,N,N',N'-tetramethyl-para-phenylenediamine
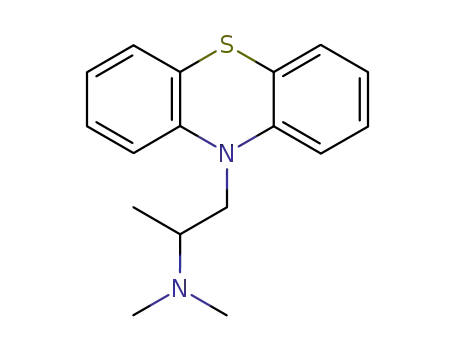
10-[2-(dimethylamino)propyl]phenothiazine
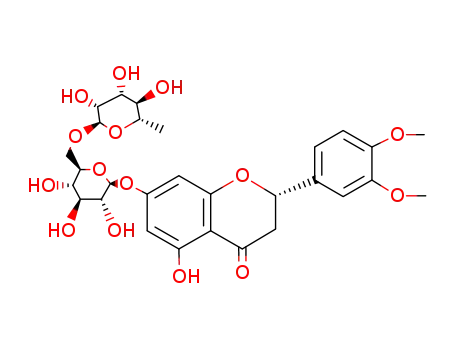
homoesperetin-7-O-rutinoside
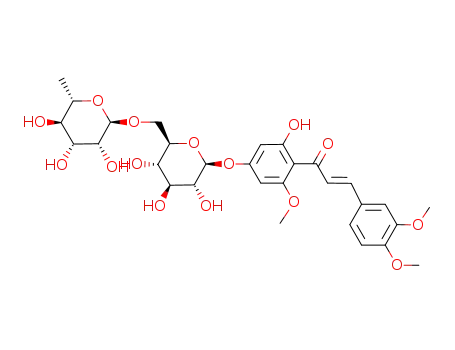
2'-hydroxy-3,4,6'-trimethoxy-4'-(O6-α-L-rhamnopyranosyl-β-D-glucopyranosyloxy)-trans-chalcone
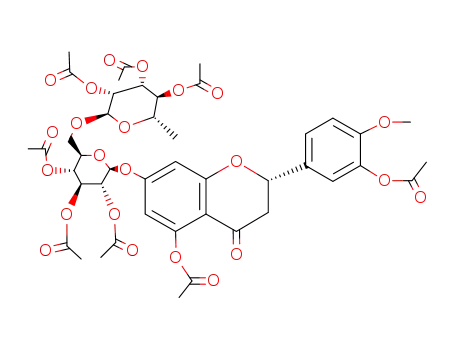
octa-O-acetylhesperidin
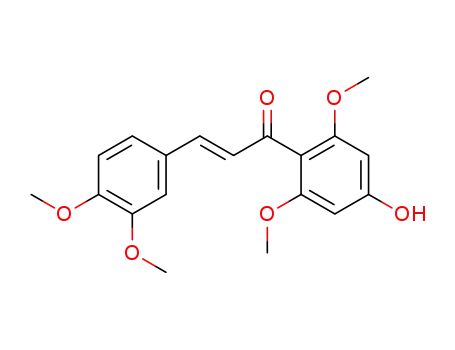
4'-hydroxy-3,4,2',6'-tetramethoxy-trans-chalcone