Your Location:Home > Products > Herbs Extract and Health Care > Fucoxanthin


CasNo: 3351-86-8
MF: C42H58O6
Appearance: Crystalline solid
|
Biochem/physiol Actions |
Xanthophyll carotenoid pigment extracted from algae. Exhibits anticancer, antioxidant, anti-obesity and anti-inflammatory properties. |
|
Definition |
ChEBI: A natural product found in Sporochnus comosus. |
|
General Description |
Fucoxanthin is isolated from brown algae. It has anti-angiogenic, hepatoprotective,?cardiovascular and cerebrovascular protective properties. Fucoxanthin stimulates G1 cell-cycle arrest and apoptosis in cancer cell lines. |
InChI:InChI=1/C42H60O7/c1-29(18-14-19-31(3)22-23-37-38(6,7)26-35(49-33(5)43)27-40(37,10)46)16-12-13-17-30(2)20-15-21-32(4)36(45)28-42(48)39(8,9)24-34(44)25-41(42,11)47/h12-22,34-35,44,46-48H,24-28H2,1-11H3/b13-12+,18-14+,20-15+,29-16+,30-17+,31-19+,32-21+/t23-,34-,35-,40+,41+,42-/m0/s1
In a comparative study, diphenyl diselen...
The first total synthesis of optically a...
The first total synthesis of optically a...
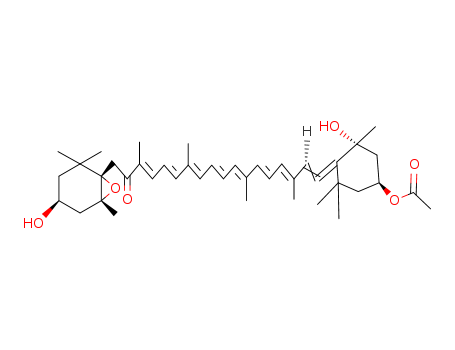
![Acetic acid (1S,3R)-3-hydroxy-4-[(3E,5E,7E,9E,11E,13E,15E)-18-((R)-4-hydroxy-2,6,6-trimethyl-cyclohex-1-enyl)-3,7,12,16-tetramethyl-17-oxo-octadeca-1,3,5,7,9,11,13,15-octaenylidene]-3,5,5-trimethyl-cyclohexyl ester](/upload/2025/8/7b4649ce-3028-4606-9446-362302a902d9.png)
Acetic acid (1S,3R)-3-hydroxy-4-[(3E,5E,7E,9E,11E,13E,15E)-18-((R)-4-hydroxy-2,6,6-trimethyl-cyclohex-1-enyl)-3,7,12,16-tetramethyl-17-oxo-octadeca-1,3,5,7,9,11,13,15-octaenylidene]-3,5,5-trimethyl-cyclohexyl ester

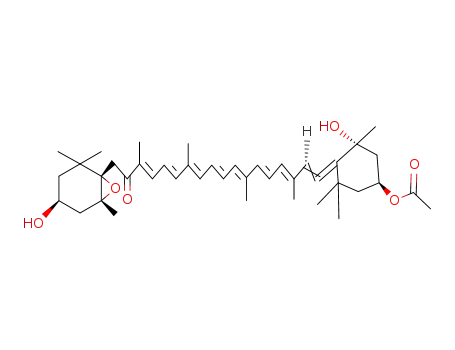
fucoxanthin

![Acetic acid (1S,3R)-3-hydroxy-4-[(3E,5E,7E,9E,11E,13E,15E)-18-((1R,4S,6S)-4-hydroxy-2,2,6-trimethyl-7-oxa-bicyclo[4.1.0]hept-1-yl)-3,7,12,16-tetramethyl-17-oxo-octadeca-1,3,5,7,9,11,13,15-octaenylidene]-3,5,5-trimethyl-cyclohexyl ester](/upload/2025/8/793593dd-8ae3-455f-8f3c-13189e6ce3ed.png)
Acetic acid (1S,3R)-3-hydroxy-4-[(3E,5E,7E,9E,11E,13E,15E)-18-((1R,4S,6S)-4-hydroxy-2,2,6-trimethyl-7-oxa-bicyclo[4.1.0]hept-1-yl)-3,7,12,16-tetramethyl-17-oxo-octadeca-1,3,5,7,9,11,13,15-octaenylidene]-3,5,5-trimethyl-cyclohexyl ester
| Conditions | Yield |
|---|---|
|
With
3-chloro-benzenecarboperoxoic acid;
|
28% 8% |
|
With
3-chloro-benzenecarboperoxoic acid;
In
dichloromethane;
at 0 ℃;
for 1h;
Yield given;
|


fucoxanthin
| Conditions | Yield |
|---|---|
|
With
iodine;
for 8h;
Irradiation;
sunlight;
|
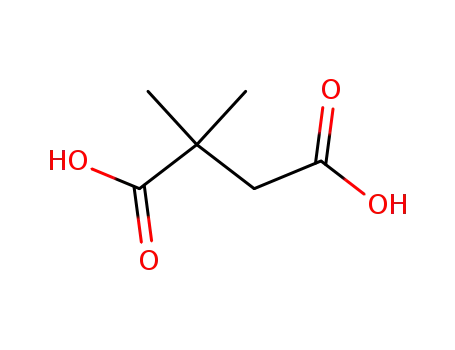
2,2-dimethylsuccinic acid
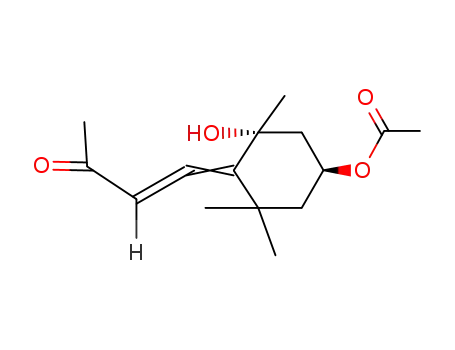
3'R,4'-<(2R,4S)-2-hydroxy-4-acetoxy-2,6,6-trimethylcyclohexylidene>but-3'-en-2'-one
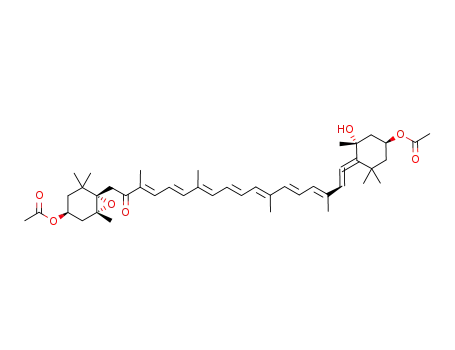
fucoxanthin monoacetate
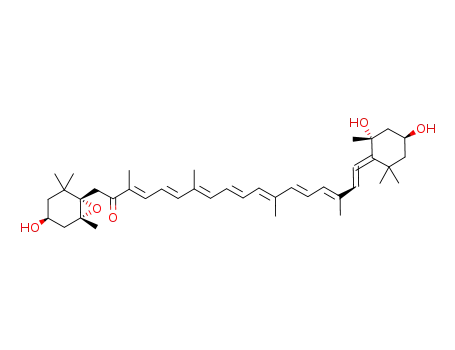
fucoxanthinol