Your Location:Home > Products > Food Ingredients > Tert-Butylhydroquinone
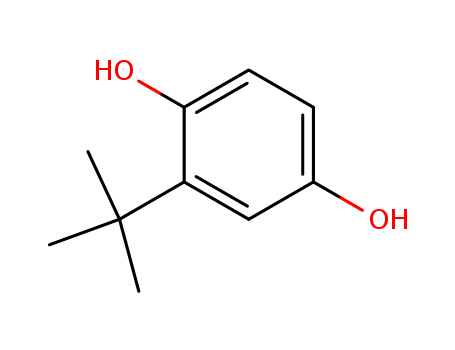


CasNo: 1948-33-0
MF: C10H14O2
Appearance: tan powder
|
Air & Water Reactions |
Insoluble in water. |
|
Reactivity Profile |
Phenols, such as tert-Butylhydroquinone, do not behave as organic alcohols, as one might guess from the presence of a hydroxyl (-OH) group in their structure. Instead, they react as weak organic acids. Phenols and cresols are much weaker as acids than common carboxylic acids (phenol has Ka = 1.3 x 10^[-10]). These materials are incompatible with strong reducing substances such as hydrides, nitrides, alkali metals, and sulfides. Flammable gas (H2) is often generated, and the heat of the reaction may ignite the gas. Heat is also generated by the acid-base reaction between phenols and bases. Such heating may initiate polymerization of the organic compound. Phenols are sulfonated very readily (for example, by concentrated sulfuric acid at room temperature). The reactions generate heat. Phenols are also nitrated very rapidly, even by dilute nitric acid. Nitrated phenols often explode when heated. Many of them form metal salts that tend toward detonation by rather mild shock. tert-Butylhydroquinone is incompatible with oxidizers. |
|
Fire Hazard |
tert-Butylhydroquinone is combustible. |
|
Flammability and Explosibility |
Nonflammable |
|
Contact allergens |
This antioxidant has seldom been reported as a sensitizer, mainly in cosmetics (lipsticks, lip-gloss, hair dyes) or in cutting oils. Simultaneous/cross-reactions have been described to butylhydroxyanisole (BHA) and less frequently to butylhydroxytoluene (BHT), but not to hydroquinone |
|
Purification Methods |
Recrystallise the hydroquinone from H2O or MeOH and dry it in a vacuum at 70o. Store it in a dark container. [Stroh et al. Angew Chem 69 699 1957, Beilstein 6 IV 6013.] |
|
Definition |
ChEBI: A member of the class of hydroquinones in which one of the ring hydrogens of hydroquinone is replaced by a tert-butyl group. |
|
General Description |
White to light tan crystalline powder or a fine beige powder. Very slight aromatic odor. |
InChI:InChI=1/C10H12O3/c1-10(2,3)8-6(11)4-5-7(12)9(8)13/h4-5,13H,1-3H3
Perfluorosulfonic acid-functionalized ca...
Covalently linked sulfonic acid (SO3H) m...
Candida antarctica lipase B proved to be...
The invention discloses a method for syn...
The preparation method 2 - of the high-p...
The invention relates to the technical f...
Palladium(II)-catalyzed oxidation reacti...
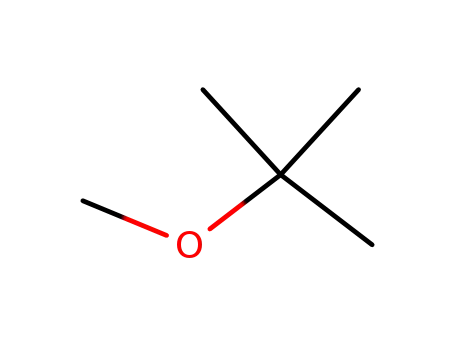
tert-butyl methyl ether

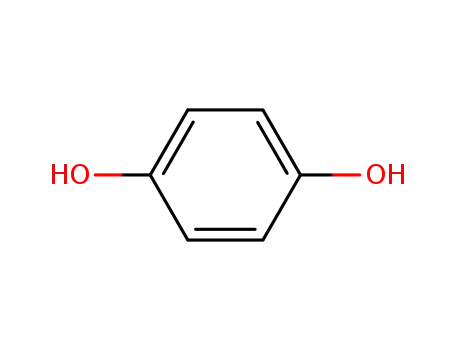
hydroquinone

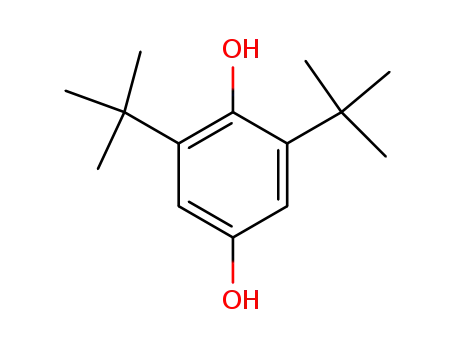
2,6-di-tert-butyl-4-hydroxyphenol

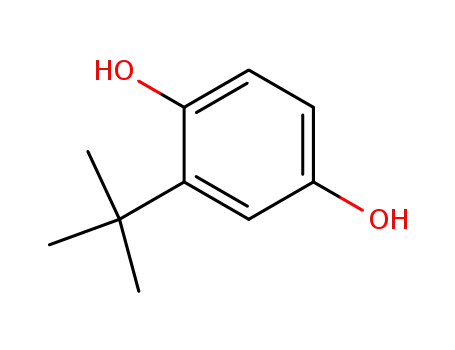
tert-butylhydroquinone
| Conditions | Yield |
|---|---|
|
With
3-(4-sulfobutylamino)propylsilanized MCM-41;
In
nitrobenzene;
at 165 ℃;
for 0.166667h;
chemoselective reaction;
Microwave irradiation;
Green chemistry;
|

tert-butyl methyl ether


hydroquinone


2,6-di-tert-butyl-4-hydroxyphenol

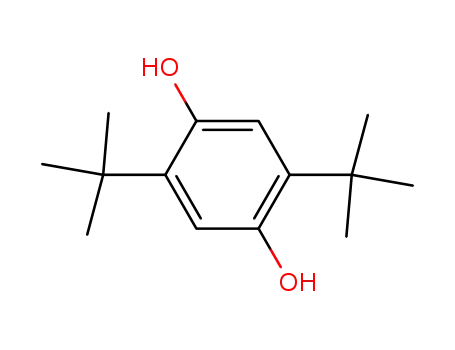
2,5-bis(1,1-dimethylethyl)-1,4-benzenediol

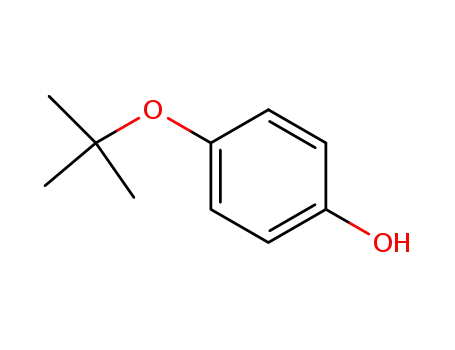
4-(tert-butoxy)phenol


tert-butylhydroquinone
| Conditions | Yield |
|---|---|
|
With
3-(4-sulfobutylamino)propylsilanized MCM-41;
In
nitrobenzene;
at 150 ℃;
for 0.133333h;
Concentration;
chemoselective reaction;
Catalytic behavior;
Microwave irradiation;
Green chemistry;
|

hydroquinone
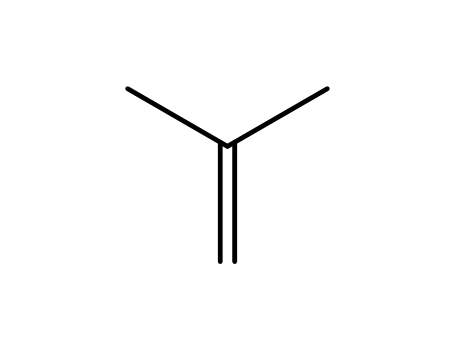
isobutene
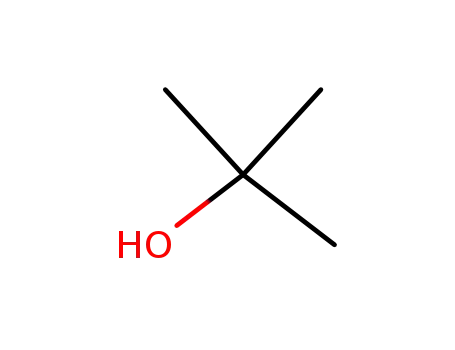
tert-butyl alcohol
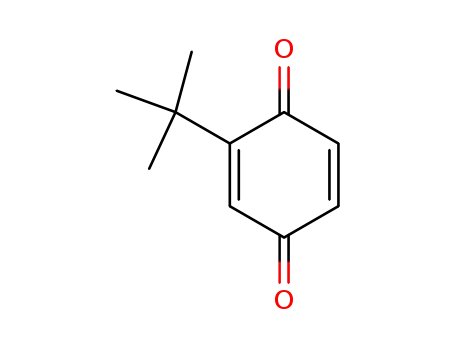
2-tert-butyl-1,4-benzoquinone
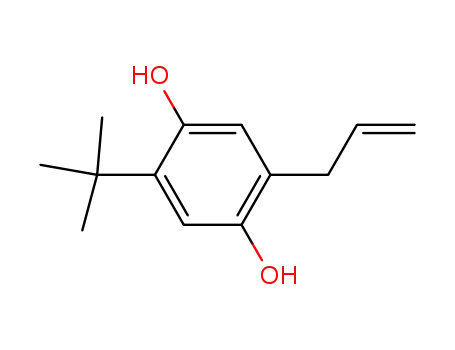
2-(1,1-dimethylethyl)-5-(2-propenyl)-1,4-benzenediol
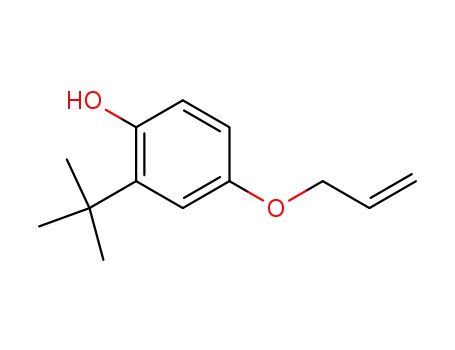
5-allyloxy-2-hydroxy-t-butylbenzene
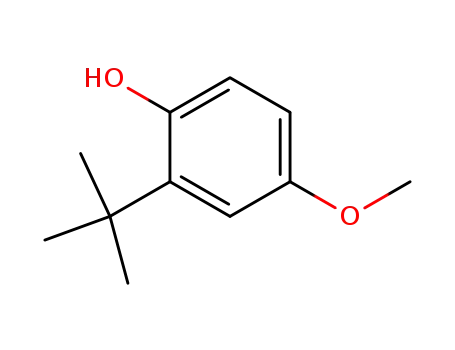
3-tert-Butyl-4-hydroxyanisole
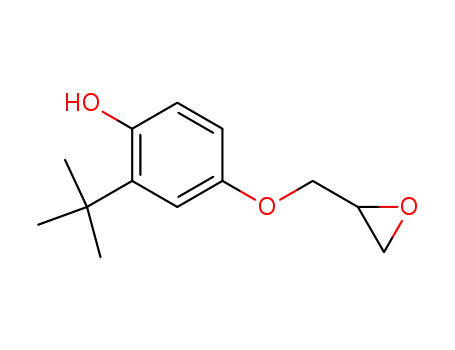
2-tert-Butyl-4-(2,3-epoxy-propoxy)-phenol