Your Location:Home > Products > Herbs Extract and Health Care > Apigenin


CasNo: 520-36-5
MF: C15H10O5
Appearance: Pale Yellow Crystalline Solid
|
Preparation |
4-hydroxybenzaldehyde (1.22 g, 9.97 mmol, 1.0 equiv) was added to asolution of 50% KOH (aq.) (6.72 g, 59.82 mmol, 6.0 equiv) and ethanol (3 mL) andstirred for 10 min. Then compound 9 (2.02g, 9.97 mmol, 1.0 equiv) was added to the reaction mixture and heated to 60 °C and stirred for 4 h. After cooled toroom temperature, the mixture was poured into ice water and acidified withconcentrated hydrochloric acid to pH = 3. Then the suspension was filtrated, washed and the residue was dried toafford Apigenin(2.43 g, 90%) as a red solid. |
|
Anticancer Research |
It induces apoptosis by targeting leptin/leptin receptor pathway and by targeting caspase-dependent extrinsic pathway aswell as STAT3 signaling pathway in lung adenocarcinoma and BT-474 breast cancercells, respectively. It shows antitumor activity against breastcancer MCF-7 cells and colon cancer HCT 116 cells and is a mediator of cancerchemoprevention and an inducer of autophagy. It can be used to treat colon canceras it induces apoptosis in colon cancer cells. It also increase melanogenesis in B16cells by activating the p38 MAPK pathway. |
|
Purification Methods |
The current method for the purification of apigenin is crude-solvent extraction method by using solvent in small quantity and also time saving. Apigenin that was isolated from Symphyotrichum novae-angliae was obtained in large quantity and directly from extract. |
InChI:InChI=1/C15H10O5/c16-9-3-1-8(2-4-9)13-7-12(19)15-11(18)5-10(17)6-14(15)20-13/h1-7,16-18H
-
Biotransformation of chrysin by Cunningh...
Natural flavonoids, such as naringenin, ...
The new flavonoid diglycoside thamiflasi...
-
A new flavone glycoside, apigenin 5-O-α-...
-
A cDNA encoding flavone synthase I was a...
Ten flavonoid glycosides were isolated a...
-
The title apigenin triglycoside was isol...
Provided is a cosmetic composition for s...
The invention discloses an 8-azacyclo-su...
-
Poly (ADP-ribose) polymerase-1 (PARP-1) ...
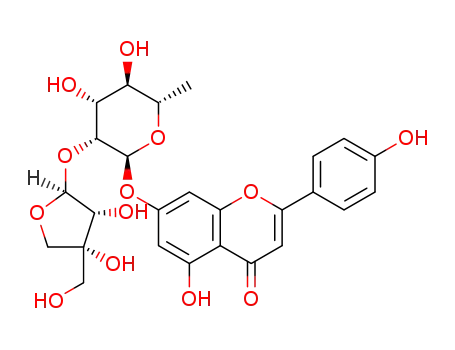
apigenin-7-O-(β-D-apiofuranosyl-(1→2)-α-L-rhamnopyranozide)

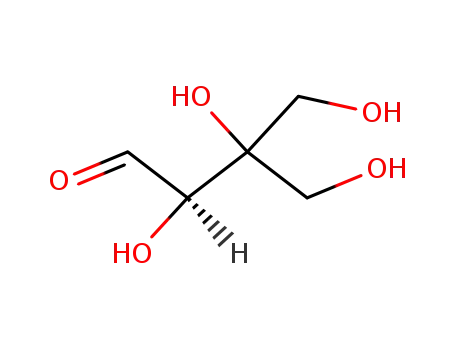
D-apiose

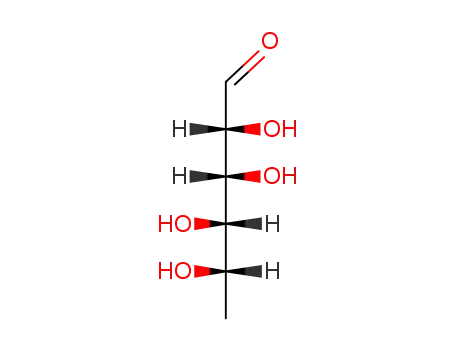
L-Rhamnose

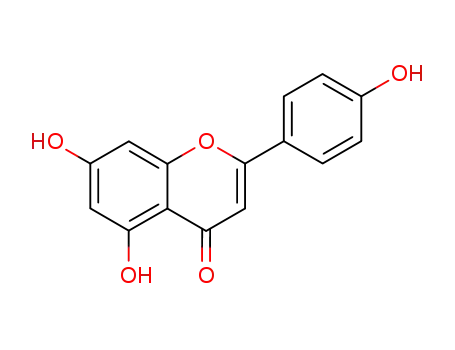
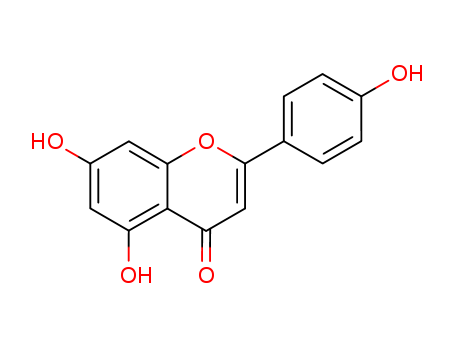
5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one
| Conditions | Yield |
|---|---|
|
With
hydrogenchloride;
In
methanol;
for 2h;
Reflux;
|
2.5 mg |
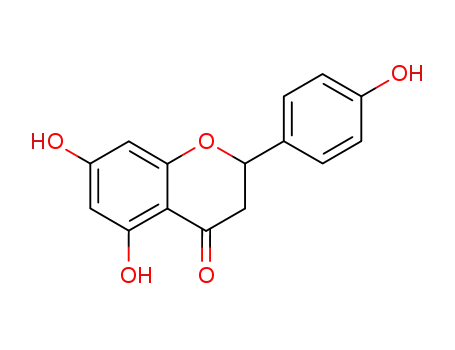
5,7-Dihydroxy-2-(4-hydroxy-phenyl)-chroman-4-on


5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one

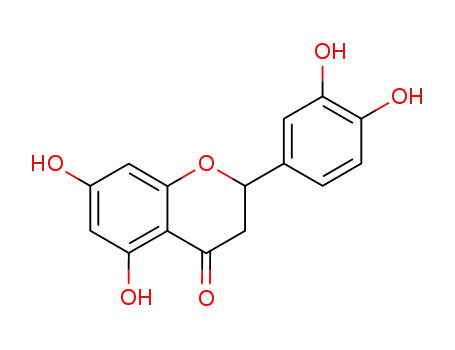
eriodictyol
| Conditions | Yield |
|---|---|
|
With
plasmid pFusionF87V-carrying recombinant Escherichia coli BL21 (DE3) cells;
at 28 ℃;
for 24h;
Microbiological reaction;
|
5.2% 3.7% |

5,7-Dihydroxy-2-(4-hydroxy-phenyl)-chroman-4-on
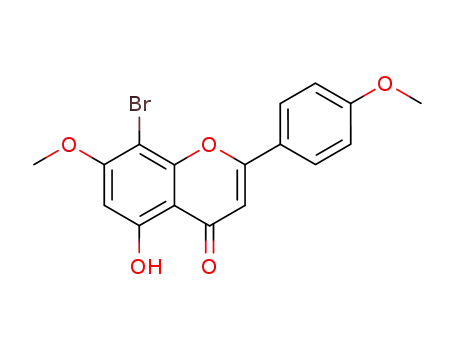
6-bromo-5-hydroxy-7,4'-dimethoxyflavone
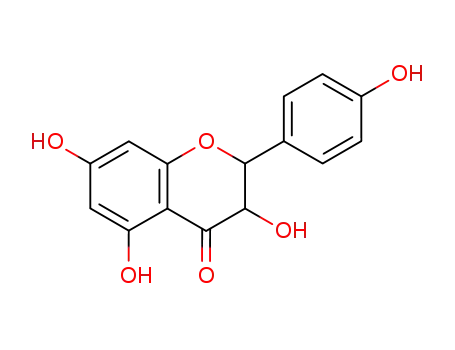
dihydrokaempferol
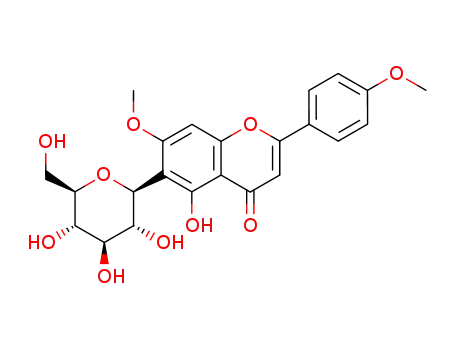
4'-O-methylapigenin 6-C-β-D-glucopyranoside
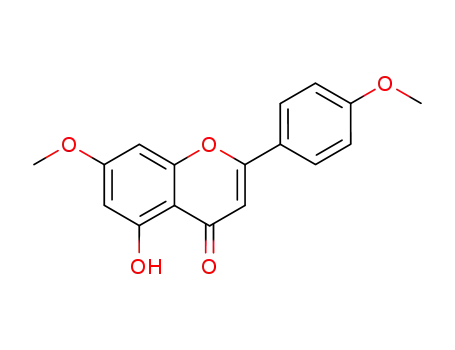
5-hydroxy-7-methoxy-2-(4-methoxyphenyl)-4H-chromen-4-one
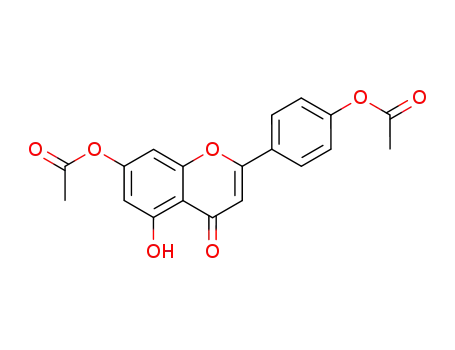
7,4'-diacetoxy-5-hydroxyflavone
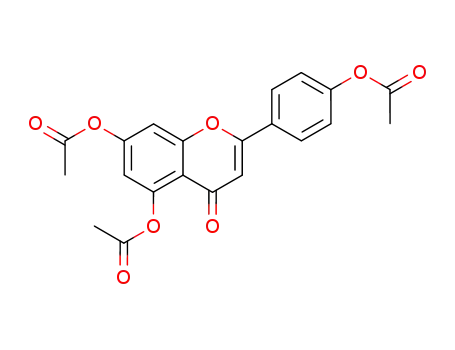
apigenin triacetate
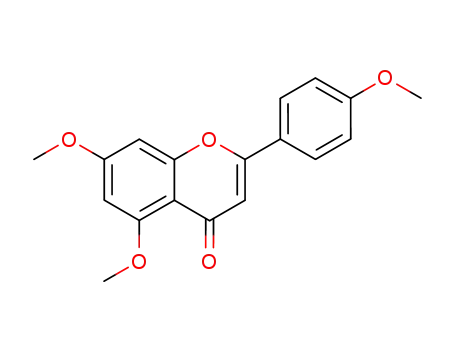
4',5,7-trimethoxyflavone