Your Location:Home > Products > Cosmetic and Beauty Materials > Allantoin
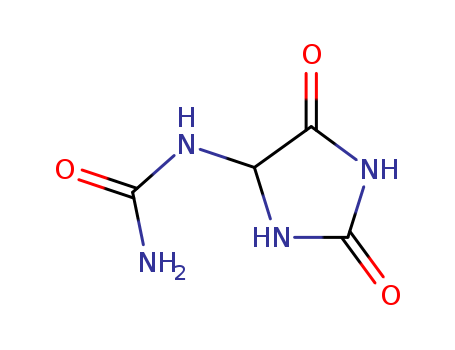


CasNo: 97-59-6
MF: C4H6N4O3
Appearance: White crystalline powder
Quality products make an important contribution to long-term revenue and profitability. Bulk supply high purity Allantoin 97-59-6, Paid sample available
allantoin is a botanical extract said to be healing, calming, and soothing, it can also help protect the skin from harmful external factors (e.g., wind burn). It is considered an excellent temporary anti-irritant and is believed to stimulate new tissue growth, helping heal damaged skin. Allantoin is appropriate for sensitive, irritated, and acne skins. Derived from the comfrey root, it is considered non-allergenic.
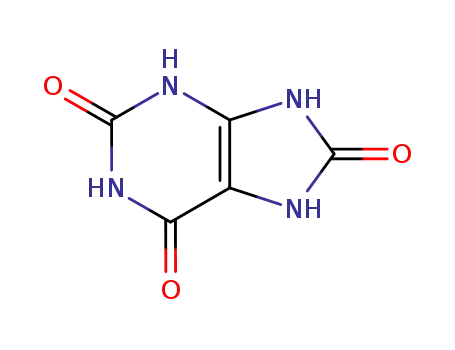
uric Acid

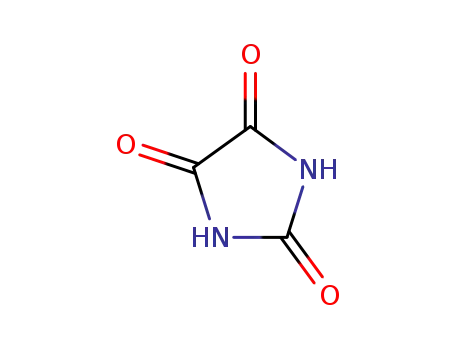
parabanic acid

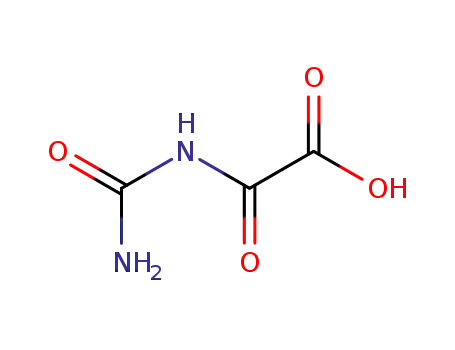
oxaluric acid

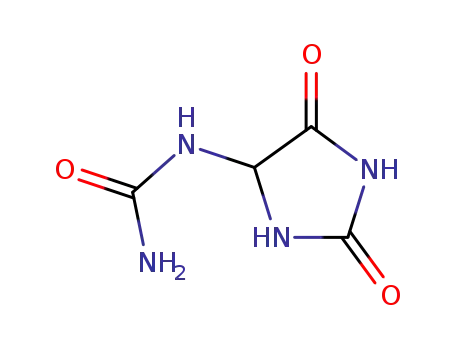
Allantoin
| Conditions | Yield |
|---|---|
|
In
water;
for 2h;
UV-irradiation;
|

uric Acid

acetic acid


oxaluric acid


Allantoin
| Conditions | Yield |
|---|---|
|
|
The CAS number of Allantoin is 97-59-6.
More information of Allantoin 97-59-6 are:
|
CAS Number |
97-59-6 |
|
Density |
1.652 g/cm3 |
|
Melting Point |
230 °C (dec.)(lit.) |
|
Boiling Point |
478oC |
|
Flash Point |
230-234 °C |
|
Refractive Index |
1.8500 (estimate) |
|
HS CODE |
29332100 |
|
PSA |
113.32000 |
|
LogP |
-0.43100 |
|
Pka |
8.96(at 25℃) |
Synonyms for Allantoin 97-59-6:(2,5-dioxoimidazolidin-4-yl)urea;Glyoxylic(acid) diureide;urea, N-(2,5-dioxo-4-imidazolidinyl)-;Cutemol emollient;[(4S)-2,5-dioxoimidazolidin-4-yl]urea;5-Ureido-2,4-imidazolidindion;[(4R)-2,5-dioxoimidazolidin-4-yl]urea;Urea, (2,5-dioxo-4-imidazolidinyl)-;Urea, (2,5-dioxo-4-imidazolidinyl)- (9CI);Glyoxyldiureide;Urea, (2, 5-dioxo-4-imidazolidinyl)-;Glyoxylic diureide;(2,5-Dioxo-4-imidazolidinyl)urea;5-Ureidohydantoin;Allantoin (5-Ureidohydantoin);Urea,(2,5-dioxo-4-imidazolidinyl)-;
The chemical formula of Allantoin is C4H6N4O3 which containing 4 Carbon atoms,6 Hydrogen atoms,4 Nitrogen atoms and 3 Oxygen atoms,and the molecular weight of Allantoin is 158.117.
Allantoin is a product of purine and uric acid metabolism. It is formed through oxidation of uric acid by urate oxidase in most mammals but is formed only through non-enzymatic oxidation by free radicals in humans. Urinary levels of allantoin are increased prior to the onset of Alzheimer’s disease symptoms in mice expressing mutations in amyloid precursor protein and tau (APP/tau) but not during the early/middle stage of the disease, indicating its potential use as a biomarker for predicting Alzheimer’s disease onset. Due to the formation of allantoin by free radicals in humans, increased urinary levels are a potential biomarker for oxidative stress status.
InChI:InChI=1/C4H6N4O3/c5-3(10)6-1-2(9)8-4(11)7-1/h1H,(H3,5,6,10)(H2,7,8,9,11)/t1-/m0/s1
Relevant articles related to Allantoin:
|
Article |
Source |
|
A statistical method for the determination of serum uric acid. |
Tawa,Etoh,Hirose , p. 3381 - 3384 (1980) |
|
Kinetics and mechanism of allantoin racemization |
Kahn, Kalju,Tipton, Peter A. , p. 62 - 72 (2000) |
Allright GC (Jinan) Biotechnology Ltd. is a quality supplier of Allantoin. Our main goal is customer satisfaction. Contact us to negotiate the best price for your business on Allantoin 97-59-6.